Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V] [W]
[X] [Y] [Z]
DARK PHOTOCHEMISTRY
(photochemistry without light)
Chemical reactions involving electronically excited molecular entities
which are generated thermally rather than by absorption of electromagnetic
radiation. The use of this term is discouraged.
DAVYDOV SPLITTING (factor-group
splitting)
The splitting of bands in the electronic or vibrational spectra of
crystals due to the presence of more than one (interacting) equivalent
molecular entity in the unit cell.
DEACTIVATION
Any loss of energy by an excited molecular entity.
See emission, energy
transfer, internal conversion,
radiationless deactivation
and transition, radiative
transition.
DELAYED FLUORESCENCE
See delayed luminescence.
DELAYED LUMINESCENCE
Luminescence decaying more slowly than that expected from the rate
of decay of the emitting state. The following mechanisms of luminescence
provide examples:
(1) triplet-triplet annihilation to form one molecular entity in its
excited singlet state and another molecular entity in its electronic
ground state (sometimes referred to as P type),
(2) thermally activated delayed fluorescence involving reversible
intersystem crossing (sometimes referred to as E type), and
(3) combination of oppositely charged ions or of an electron and a
cation. For emission to be referred to in this case as delayed luminescence
at least one of the two reaction partners must be generated in a photochemical
process.
DEDMR
See ODMR.
DEPTH OF PENETRATION
(of light)
The inverse of the absorption coefficient. The SI unit is m. If the
decadic absorption coefficient, a, is used, the depth of penetration
(1/a) is the distance at which the spectral radiant power, Pl
decreases to one tenth of its incident value,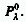 .
If the Napierian absorption coefficient,
.
If the Napierian absorption coefficient, 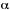 ,
is used, the depth of penetration (1/
,
is used, the depth of penetration (1/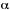 =
= 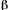 in this case) is the distance at which the spectral radiant power
decreases to 1/e of its incident value.
in this case) is the distance at which the spectral radiant power
decreases to 1/e of its incident value.
See absorbance, attenuance
DEXTER EXCITATION
TRANSFER (Electron Exchange Excitation Transfer)
Excitation transfer occurring as a result of an electron exchange
mechanism. It requires an overlap of the wavefunctions of the energy
donor and the energy acceptor. It is the dominant mechanism in triplet-triplet
energy transfer. The transfer rate constant, kET, is given
by
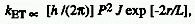 ,
,
where r is the distance between donor (D) and acceptor
(A), L and P are constants not easily related to experimentally determinable
quantities, and J is the spectral overlap integral. For this mechanism
the spin conservation rules are obeyed.
See also radiative
energy transfer.
DFDMR
See ODMR.
DIABATIC ELECTRON
TRANSFER
Electron transfer process in which the reacting system has to cross
over between different electronic surfaces in passing from reactants
to products. For diabatic electron transfer the electronic transmission
factor is << 1 (see Marcus equation.) The term non-adiabatic
electron transfer has also been used and is in fact more widespread,
but should be discouraged because it contains a double negation.
See also adiabatic
electron transfer
DIABATIC PHOTOREACTION
Within the Born-Oppenheimer approximation, a reaction beginning on
one excited state "potential-energy surface" and ending, as a result
of radiationless transition, on another surface, usually that of the
ground state. Also called non-adiabatic.
Compare with adiabatic photoreaction.
DIODE LASERS
Sources of CW or pulsed coherent radiation in the visible and infrared
regions. These lasers are semiconductor devices of small dimensions.
Also called semiconductor lasers.
DI- -METHANE
REARRANGEMENT
-METHANE
REARRANGEMENT
A photochemical reaction of a molecular entity comprising two  -systems,
separated by a saturated carbon atom (a 1,4-diene or an allyl-substituted
aromatic analog), to form an ene- (or aryl-) substituted cyclopropane.
The rearrangement formally amounts to a 1,2 shift of one ene group
(in the diene) or the aryl group (in the allyl-aromatic analog) and
"bond formation" between the lateral carbons of the nonmigrating moiety.
-systems,
separated by a saturated carbon atom (a 1,4-diene or an allyl-substituted
aromatic analog), to form an ene- (or aryl-) substituted cyclopropane.
The rearrangement formally amounts to a 1,2 shift of one ene group
(in the diene) or the aryl group (in the allyl-aromatic analog) and
"bond formation" between the lateral carbons of the nonmigrating moiety.
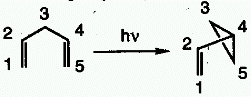
See also oxa-di- -methane
rearrangement.
-methane
rearrangement.
DIPOLAR MECHANISM (of
energy transfer)
Same as Förster excitation transfer.
See also energy transfer.
DIPOLE-DIPOLE
EXCITATION TRANSFER
Same as Förster excitation transfer.
See also energy transfer.
DIRADICAL
This term, synonymous with biradical, is no longer recommended.
DOSE
The energy or amount of photons absorbed per unit area or unit volume
by an irradiated object during a particular exposure time. In medicine
and in some other research areas (e.g. photopolymerization and water
handling through irradiation) dose is used in the sense of fluence,
i.e. the energy or amount of photons per unit area or unit volume
received by an irradiated object during a particular exposure time.
The SI units are J m-2 or J m-3 and mol m-2
or mol m-3, respectively.
See also UV-dose.
DOUBLET STATE
A state having a total electron spin quantum number equal to 1/2.
See multiplicity.
DRIVING FORCE (for electron
transfer)
Term widely used to indicate the negative of the standard Gibbs energy
change (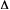 Go)
for (photoinduced) outer-sphere electron transfer . This quantity
can often be calculated rather accurately from independently determined
properties of the donor and acceptor species involved. Thus e.g. for
photoinduced electron transfer between a neutral acceptor (A) and
a neutral donor (D) (either one of them may be the electronically
excited molecular entity) to form an ion pair, the driving force in
a solvent with static dielectric constant
Go)
for (photoinduced) outer-sphere electron transfer . This quantity
can often be calculated rather accurately from independently determined
properties of the donor and acceptor species involved. Thus e.g. for
photoinduced electron transfer between a neutral acceptor (A) and
a neutral donor (D) (either one of them may be the electronically
excited molecular entity) to form an ion pair, the driving force in
a solvent with static dielectric constant 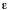 s
can be approximated as (see: A. Weller, Z. Phys. Chem. Neue Folge
133, 93-98 (1982)):
s
can be approximated as (see: A. Weller, Z. Phys. Chem. Neue Folge
133, 93-98 (1982)):

with Eo(D/D+) the standard oxidation
potential of the donor, Eo(A/A-) the standard
reduction potential of the acceptor, )
the change in Gibbs energy for bringing the two radical ions to an
encounter distance
)
the change in Gibbs energy for bringing the two radical ions to an
encounter distance 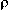 ,
and
,
and 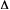 Eo,o
the electronic excitation energy of the excited partner, all data
referring to the same solvent.
Eo,o
the electronic excitation energy of the excited partner, all data
referring to the same solvent.
DYE LASER
A CW or pulsed source of coherent radiation in which the active medium
is usually a solution of a fluorescent organic molecule (the dye)
pumped with a suitable pump laser or with a flash lamp. These lasers
can be tuned over a large part of the fluorescence band of the dye.
DYNAMIC QUENCHING
See quenching.

