Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V] [W]
[X] [Y] [Z]
RADIANCE (L)
For a parallel beam it is the radiant power, P, of all wavelengths
leaving or passing through an infinitesimal element of surface in
a given direction from the source divided by the orthogonally projected
area of the element in a plane normal to the given direction of the
beam, 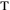 ,
[(dP/dS)/ cos
,
[(dP/dS)/ cos 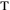 ,
simplified expression: L = P/(S cos
,
simplified expression: L = P/(S cos 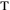 )
when the radiant power is constant over the surface area considered].
The SI unit is W m-2. Note that
)
when the radiant power is constant over the surface area considered].
The SI unit is W m-2. Note that 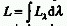 , where
, where 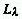 is the spectral radiance
at wavelength
is the spectral radiance
at wavelength  .
For a divergent beam propagating in an elementary cone of the solid
angle dw containing the given direction q, the radiance is d2P/(d
.
For a divergent beam propagating in an elementary cone of the solid
angle dw containing the given direction q, the radiance is d2P/(d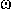 dS cos
dS cos 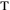 ),
with SI units W m-2 sr-1.
),
with SI units W m-2 sr-1.
See also photon flow,
photon radiance, spectral
radiance, spherical radiance
.
RADIANT EMITTANCE
See radiant exitance.
RADIANT ENERGY (Q)
The total energy emitted, transferred or received as radiation of
all wavelengths in a defined period of time 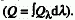 .
It is the product of radiant power, P, and time, t: Q = P t when the
radiant power is constant over the time considered. The SI unit is
J.
.
It is the product of radiant power, P, and time, t: Q = P t when the
radiant power is constant over the time considered. The SI unit is
J.
See also spectral
radiant power.
RADIANT (ENERGY) FLUX
(P,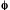 )
)
Although flux is generally used in the sense of the 'rate of transfer
of fluid, particles or energy across a given surface', the radiant
energy flux has been adopted by IUPAC as equivalent to radiant power,
P. (P = 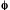 = dQ/d
= dQ/d ,
simplified expression: P =
,
simplified expression: P = 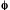 = Q/t when the radiant energy, Q, is constant over the time considered).
In photochemistry
= Q/t when the radiant energy, Q, is constant over the time considered).
In photochemistry 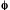 is reserved for quantum yield.
is reserved for quantum yield.
See alsophoton flow,
photon radiance, , radiant
energy, spectral radiant
flux
RADIANT EXITANCE (M)
The radiant power, P, emitted at all wavelengths by an element of
the surface containing the source point under consideration divided
by the surface area (S) of that element. (dP/dS, simplified expression:
M = P/S when the radiant power is constant over the surface area considered).
It is the integration of the radiant power leaving a source over the
solid angle and over the whole wavelength range. The SI unit is W
m-2. Note that 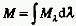 ,
where
,
where 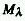 is the spectral radiant
exitance at wavelength
is the spectral radiant
exitance at wavelength  .
Formerly called radiant emittance. Same as spherical radiant exitance.
.
Formerly called radiant emittance. Same as spherical radiant exitance.
See also photon exitance,
spectral radiant exitance.
RADIANT EXPOSURE (H)
The irradiance, E, integrated over the time of irradiation  simplified
expression H = E t when the irradiance is constant over the time considered).
The SI unit is J m-2. For a parallel and perpendicularly
incident beam not scattered or reflected by the target or its surroundings
fluence (H0) is an equivalent term.
simplified
expression H = E t when the irradiance is constant over the time considered).
The SI unit is J m-2. For a parallel and perpendicularly
incident beam not scattered or reflected by the target or its surroundings
fluence (H0) is an equivalent term.
RADIANT INTENSITY (I)
Radiant (energy) flux or radiant power, P, at all wavelenghts per
unit solid angle, 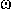 .
The radiant power emitted in a given direction by a source or an element
of the source in an infinitesimal cone containing the given direction
divided by the solid angle of the cone (dP/d
.
The radiant power emitted in a given direction by a source or an element
of the source in an infinitesimal cone containing the given direction
divided by the solid angle of the cone (dP/d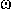 ,
simplified expression: I = P/S when the radiant power is constant
over the surface area considered). The SI unit is W sr-1.Note
that
,
simplified expression: I = P/S when the radiant power is constant
over the surface area considered). The SI unit is W sr-1.Note
that 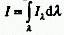 , where
, where 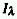 is
the spectral radiant intensity at wavelength
is
the spectral radiant intensity at wavelength  .
.
See also spectral
radiant intensity.
RADIANT POWER (P)
Same as radiant (energy) flux, 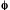 .
Power emitted, transferred or received as radiation. The SI unit is
J s-1 = W.
.
Power emitted, transferred or received as radiation. The SI unit is
J s-1 = W.
See spectral
radiant power.
RADIATIONLESS
DEACTIVATION (Decay)
Loss of electronic excitation energy without photon emission or chemical
change.
See energy transfer,
internal conversion, intersystem
crossing.
RADIATIONLESS TRANSITION
A transition between two states of a system without photon emission
or absorption.
Compare radiative transition.
RADIATIVE ENERGY
TRANSFER
Transfer of excitation energy by radiative deactivation of a donor
molecular entity and reabsorption of the emitted light by an acceptor
molecular entity. The probability of transfer is given approximately
by
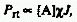 ,
,
where J is the spectral overlap integral, [A] is the
concentration of the acceptor, and 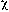 is the specimen thickness. This type of energy transfer depends on
the shape and size of the vessel utilized. Same as trivial energy
transfer.
is the specimen thickness. This type of energy transfer depends on
the shape and size of the vessel utilized. Same as trivial energy
transfer.
See also Dexter
excitation transfer, energy transfer,
Förster excitation
transfer.
RADIATIVE LIFETIME
( 0)
0)
The lifetime of an excited molecular entity in the absence of radiationless
transitions. It is the reciprocal of the first-order rate constant
for the radiative step, or of the sum of these rate constants if there
is more than one such step. The equivalent term, natural lifetime,
is discouraged. Approximate expressions exist relating  0
to the oscillator strength of the emitting transition.
0
to the oscillator strength of the emitting transition.
RADIATIVE TRANSITION
A transition between two states of a molecular entity, the energy
difference being emitted or absorbed as a photon.
See luminescence.
Compare radiationless deactivation, radiationless transition.
RADICAL PAIR
Two radicals in close proximity, usually within a solvent "cage" or
at least sufficiently close to allow spin correlation. The radicals
may be formed simultaneously by some unimolecular process, e.g., photochemical
bond breaking, or they may have come together by diffusion. A radical
pair is called geminate radical pair provided that each radical partner
is a descendant of the same parental pair.
RADIOLUMINESCENCE
Luminescence arising from excitation by high energy particles or radiation.
RADIOLYSIS
Bond cleavage induced by high-energy radiation. The term is also more
loosely used for any chemical process brought about by high-energy
radiation. The term has also been used to refer to the irradiation
technique itself ("pulse radiolysis").
RED SHIFT
Informal term for bathochromic shift.
RELATIVE SPECTRAL
RESPONSIVITY 
See action spectrum.
RELAXATION
Passage of an excited or otherwise perturbed system towards or into
thermal equilibrium with its environment.
See radiationless deactivation, radiationless transition,
radiative transition.
RENNER-TELLER EFFECT
Splittings in the vibrational levels of molecular entities due to
even terms in the vibronic perturbation expansion. This is generally
a minor effect for nonlinear molecular entities compared to the Jahn-Teller
effect which is due to the odd terms. For linear molecular entities
it is the only possible vibronic effect characteristic of degenerate
electronic states.
REORGANIZATION ENERGY
(in electron transfer)
Defined as the Gibbs energy dissipated when a system that has undergone
"vertical" electron transfer (i.e. electron transfer obeying the Franck
Condon principle ) relaxes to the equilibrium state for its new charge
distribution. Commonly the total reorganization energy ( )
is written as the sum of an inner contribution (
)
is written as the sum of an inner contribution ( in
) and an outer contribution (
in
) and an outer contribution ( out
) attributed to nuclear reorganizations of the redox partners and
their environment (solvent) respectively.
out
) attributed to nuclear reorganizations of the redox partners and
their environment (solvent) respectively.
RESONANCE ABSORPTION
TECHNIQUE
The monitoring of atoms or radicals generated in the gas phase by
observing the attenuation of the radiation from a lamp emitting the
characteristic resonance radiation of the observed species.
RESONANCE FLUORESCENCE
Fluorescence from the primary excited atomic or molecular species
at the wavelength of the exciting radiation (no relaxation within
the excited manifold).
This term is also used to designate the radiation emitted by an atom
of the same wavelength as the longest one capable of exciting its
fluorescence, e.g. 122.6 nm in the case of the hydrogen atom, and
253.7 nm in the case of the mercury atom.
See also resonance line.
RESONANCE
FLUORESCENCE TECHNIQUE
The monitoring of atoms or radicals generated in the gas phase by
observing the intensity of fluorescence (exitance) emitted by the
species after excitation with radiation of the same wavelength.
RESONANCE LAMP
A lamp emitting resonance radiation of atoms and their ions. Depending
on the requirements the lamp is filled either with pure vapour of
the element or with a mixture of it and other gases. E.g., Hg (253.7
nm), Cd (228.8 and 643.8 nm), Na (589.0 nm), Zn (213.8, 330.0, 334.5,
and 636.2 nm), Kr (116.5 and 123.6 nm), Xe (129.6 and 147.0 nm).
RESONANCE LINE
The longest wavelength capable of exciting fluorescence in an atom.
See also resonance
fluorescence.
RESONANCE RADIATION
Same as resonance fluorescence.
ROVIBRONIC STATE
A state corresponding to a particular rotational sublevel of a particular
vibrational level of a particular electronic state.
RUBY LASER
A pulsed source of coherent radiation emitting mainly at 694.3 nm
from chromium ions (Cr+3) in aluminum oxide.
See laser, solid
state lasers.
RYDBERG ORBITAL
For an atom, an orbital with principal quantum number greater than
that of any occupied orbital of the ground state. For a molecular
entity, a molecular orbital which correlates with a Rydberg atomic
orbital in an atomic fragment produced by dissociation. Typically,
the extension of the Rydberg orbital is large compared to the size
of the atom or molecular entity.
RYDBERG
TRANSITION
An electronic transition described approximately as promotion of an
electron from a "bonding" orbital to a Rydberg orbital. Spectral bands
corresponding to Rydberg transitions approximately fit the Rydberg
formula
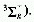
where s is the wavenumber, I the ionization potential
of the atom or molecular entity, n a principal quantum number, R the
Rydberg constant, and D the quantum defect which differentiates between
s, p, d, etc., orbitals. The notation used is, e.g., 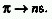 .
.
RYDMR
See ODMR.

