Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V] [W]
[X] [Y] [Z]
ABSORBANCE (A)
The logarithm to the base 10 of the ratio of the spectral radiant
power of incident , essentially monochromatic, radiation 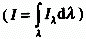 to the radiant power of transmitted radiation (Pl):
to the radiant power of transmitted radiation (Pl):
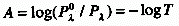
In practice, absorbance is the logarithm to the base
10 of the ratio of the spectral radiant power of light transmitted
through the reference sample to that of the light transmitted through
the solution, both observed in identical cells. T is the (internal)
transmittance. This definition supposes that all the incident light
is either transmitted or absorbed, reflection or scattering being
negligible. Traditionally (spectral )radiant intensity, I, was used
instead of spectral radiant power,Pl
, which is now the accepted form. (The terms: absorbancy, extinction,
and optical density should no longer be used.)
See absorption
coefficient, absorptance, attenuance,
Beer-Lambert law, depth
of penetration, internal
transmittance, Lambert law, molar
absorption coefficient.
ABSORPTANCE
The fraction of light absorbed, equal to one minus the transmittance
(T).
See absorbance.
ABSORPTION (of electromagnetic
radiation )
The transfer of energy from an electromagnetic field to a molecular
entity.
ABSORPTION COEFFICIENT
(decadic-a or Napierian-a)
Absorbance divided by the optical pathlength, l :
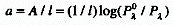
Physicists usually use natural logarithms. In this
case:
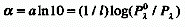
where 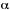 is the Napierian absorption coefficient. Since absorbance is a dimensionless
quantity, the coherent SI unit for a and
is the Napierian absorption coefficient. Since absorbance is a dimensionless
quantity, the coherent SI unit for a and 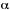 is m-1. Also cm-1 is often used.
is m-1. Also cm-1 is often used.
See also absorptivity,
molar
absorption coefficient.
ABSORPTION CROSS
SECTION (s)
Operationally, it can be calculated as the absorption coefficient
divided by the number of molecular entities contained in a unit volume
of the absorbing medium along the light path:
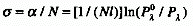
where N is the number of molecular entities per unit
volume, l is the optical pathlength, and 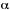 is the Napierian absorption coefficient. The relation between the
absorption cross section and the molar (decadic) absorption coefficient,
is the Napierian absorption coefficient. The relation between the
absorption cross section and the molar (decadic) absorption coefficient,
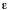 ,
(units M-1cm-1) is
,
(units M-1cm-1) is
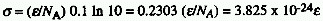
where 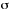 is in m2 and NA is the Avogadro constant.
is in m2 and NA is the Avogadro constant.
See attenuance, Beer-Lambert
law.
ABSORPTIVITY
Absorptance divided by the optical pathlength. For very low attenuance
it approximates the absorption coefficient (within the approximation
(1 - e-A)~ A). The use of this term is not recommended.
ACTINOMETER
A chemical system or physical device which determines the number of
photons in a beam integrally or per unit time. This name is commonly
applied to devices used in the ultraviolet and visible wavelength
ranges. For example, solutions of iron(III) oxalate can be used as
a chemical actinometer, while bolometers, thermopiles, and photodiodes
are physical devices giving a reading that can be correlated to the
number of photons detected.
ACTION SPECTRUM
A plot of a relative biological or chemical photoresponse (=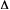 y)
per number of incident photons, against wavelength or energy of radiation
under the same radiant power of light. This form of presentation is
frequently used in the studies of biological or solid state systems,
where the nature of the absorbing species is unknown. The action spectrum
is sometimes called spectral responsivity or sensitivity spectrum.
The precise action spectrum is a plot of the spectral (photon or quantum)
effectiveness. By contrast, a plot of the biological or chemical change
or response per absorbed photon (quantum efficiency) versus wavelength
is the efficiency spectrum.
y)
per number of incident photons, against wavelength or energy of radiation
under the same radiant power of light. This form of presentation is
frequently used in the studies of biological or solid state systems,
where the nature of the absorbing species is unknown. The action spectrum
is sometimes called spectral responsivity or sensitivity spectrum.
The precise action spectrum is a plot of the spectral (photon or quantum)
effectiveness. By contrast, a plot of the biological or chemical change
or response per absorbed photon (quantum efficiency) versus wavelength
is the efficiency spectrum.
See also excitation
spectrum., efficiency spectrum
ADIABATIC ELECTRON
TRANSFER
Electron transfer process in which the reacting system remains on
a single electronic surface in passing from reactants to products.
For adiabatic electron transfer the electronic transmission factor
is close to unity (see Marcus equation.)
See also diabatic
electron transfer.
ADIABATIC PHOTOREACTION
Within the "Born-Oppenheimer approximation", a reaction of an
excited state species that occurs on a single "potential-energy surface".
Compare diabatic photoreaction.
ADMR
See ODMR.
ALPHA-CLEAVAGE (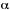 -Cleavage)
-Cleavage)
Homolytic cleavage of a bond connecting an atom or group to a specified
group. Often applied to a bond connected to a carbonyl group, in which
case it is called a Norrish type I photoreaction. This reaction should
be distinguished from an alpha-(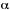 -)expulsion.
-)expulsion.
ALPHA-EXPULSION (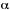 -Expulsion)
-Expulsion)
A general reaction by which a group attached to the alpha carbon of
an excited chromophore is expelled either as an odd electron species
or as an anionic species. This reaction should be distinguished from
an alpha-(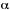 -)cleavage.
-)cleavage.
AM (0) SUNLIGHT
The solar irradiance in space just above the earth atmosphere (air
mass, AM, zero). Also called extraterrestrial global irradiance.
AM (1) SUNLIGHT
The solar irradiance traversing the atmosphere when the sun is in
a position perpendicular to the earth surface. Also called terrestrial
global irradiance.
See also AM (0) sunlight.
ANNIHILATION
Two atoms or molecular entities both in an excited electronic state
interact often (usually upon collision) to produce one atom or molecular
entity in an excited electronic state and another in its ground electronic
state. This phenomenon is sometimes referred to as energy pooling.
See singlet-singlet
annihilation, spin-conservation
rule, triplet-triplet
annihilation.
ANTIMONY-XENON LAMP
(Arc)
An intense source of ultraviolet, visible, and near infra-red radiation
produced by an electrical discharge in a mixture of antimony vapour
and xenon under high pressure. Its output in the ultraviolet region
is higher than that of the mercury-xenon arc.
See lamp.
ANTI-STOKES SHIFT
See Stokes shift.
APPARENT LIFETIME
See lifetime.
ARGON ION LASER
A CW or pulsed laser emitting lines from 334 to 529 nm from singly
ionized argon. Principal emissions are at 488.0 and 514.5 nm.
See laser, gas
lasers.
ATTENUANCE (D)
The logarithm to the base 10 of the ratio of the transmittance
(T):
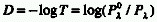
Attenuance reduces to absorbance if the incident beam
is only either transmitted or absorbed, but not reflected or scattered.
See Beer-Lambert law,
depth of penetration.
ATTENUANCE FILTER
An optical device (filter) which reduces the radiant power of a light
beam by a constant factor over all wavelengths within its operating
range. Sometimes called attenuator or neutral density filter.
AUXOCHROME
An atom or group which, when added to or introduced into a chromophore,
causes a bathochromic shift and/or a hyperchromic effect in a given
band of the chromophore, usually in that of lowest frequency. This
term is obsolete.
AVOIDED CROSSING (of
potential-energy surfaces)
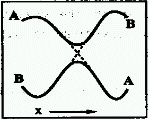
Frequently, two Born-Oppenheimer electronic states
(A,B) change their energy order as molecular geometry (x) is changed
continuously along a path. In the process their energies may become
equal at some points (the surfaces are said to cross, dotted lines
in the figure), or only come relatively close (the crossing of the
surfaces is said to be avoided). If the electronic states are of the
same symmetry, the surface crossing is always avoided in diatomics
and usually avoided in polyatomics.
Same as intended crossing.

