Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V]
[W] [X] [Y]
[Z]
MARCUS EQUATION (for
electron transfer)
Equation proposed by R.A. Marcus to relate the rate of outer-sphere
electron transfer with the thermodynamics of this process (see: R.A.
Marcus, J. Chem. Phys. 24, 966-978 (1956)). Essentially the rate constant
within the encounter complex (or the rate constant of intramolecular
transfer ) is given by the Eyring equation:
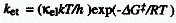
where k represents Boltzmann's constant and kel
is the so called electronic transmission factor ( kel~
1 for adiabatic and << 1 for diabatic electron transfer ). It
was shown by Marcus that for outer-sphere electron transfer the barrier
height can then be expressed as:
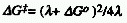
where 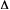 Go
represents the standard Gibbs energy change accompanying the reaction
and
Go
represents the standard Gibbs energy change accompanying the reaction
and  the total reorganization energy.
the total reorganization energy.
It should be noted that whereas the classical Marcus
equation has been found to be quite adequate in the normal region,
it is now generally accepted that in the inverted region a more elaborate
formulation, taking into account explicitly the Franck-Condon factor
due to quantum mechanical vibration modes, should be employed
MARCUS INVERTED REGION
(for electron transfer)
See inverted region
MARCUS-HUSH RELATIONSHIP
Relationship between the barrier 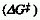 to thermal electron transfer, the energy of a corresponding optical
charge transfer transition (
to thermal electron transfer, the energy of a corresponding optical
charge transfer transition ( Eop
), and the overall change in standard Gibbs energy accompanying thermal
electron transfer (
Eop
), and the overall change in standard Gibbs energy accompanying thermal
electron transfer ( Go
). Assuming a quadratic relation between the energy of the system
and its distortions from equilibrium (harmonic oscillator model) the
expression obtained is:
Go
). Assuming a quadratic relation between the energy of the system
and its distortions from equilibrium (harmonic oscillator model) the
expression obtained is:
The simplest form of this expression obtains for degenerate
electron transfer (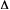 Go
) in e.g. symmetrical mixed valence systems:
Go
) in e.g. symmetrical mixed valence systems:
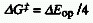
Note that for this situation the Marcus equation reads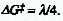 .
.
See Marcus equation
MEDIUM-PRESSURE
MERCURY LAMP (Arc)
Radiation source containing mercury vapour at pressures ranging from
100 to several hundred kPa (1 atm = 101.325 kPa). Emits mostly from
310 to 1000 nm with most intense lines at 300, 303, 313, 334, 366,
405, 436, 546, and 578 nm.
See lamp.
MERCURY-XENON LAMP
(Arc)
An intense source of ultraviolet, visible, and near infrared radiation
produced by an electrical discharge in a mixture of mercury vapour
and xenon under high pressure.
See lamp.
MERRY-GO-ROUND REACTOR
(Turntable Reactor)
An apparatus in which several samples are rotated around a radiation
source in order to expose each to equal amounts of radiation.
METAL
TO LIGAND CHARGE TRANSFER (MLCT) TRANSITION
An electronic transition of a metal complex that corresponds to excitation
populating an electronic state in which considerable electron transfer
from the metal to a ligand has occurred.
Compare ligand to metal charge transfer transition
METAL
TO METAL CHARGE TRANSFER (MMCT) TRANSITION
An electronic transition of a bi- or poly-nuclear metal complex that
corresponds to excitation populating an electronic state in which
considerable electron transfer between two metal centres has occurred.
See also intervalence
charge transfer .
MLCT
See
metal to ligand charge transfer (mlct) transition.
MODE-LOCKED LASER
A laser in which many resonant modes are coupled in phase, to yield
a train of very short pulses (e. g. ps pulses). The coupling of the
modes is obtained by modulation of the gain in the resonator, and
can be active (electro-optic modulation of the losses or of the pump
intensity), or passive (with a saturable absorber).
See also free-running
laser.
MOLAR
ABSORPTION COEFFICIENT, MOLAR DECADIC ABSORPTION COEFFICIENT
Absorbance divided by the absorption pathlength, l and the concentration,
c:
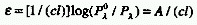
In common usage for l in cm and c in mol dm-3
or M, 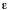 results in dm3 mol-1 cm-1 or M-1
cm-1, which equals 0.1 m2 mol-1 (coherent
SI units) = 103 cm2 mol-1 = cm2
mmol-1 = dm3 cm-1 mol-1.
The term molar absorptivity for molar absorption coefficient should
be avoided.
results in dm3 mol-1 cm-1 or M-1
cm-1, which equals 0.1 m2 mol-1 (coherent
SI units) = 103 cm2 mol-1 = cm2
mmol-1 = dm3 cm-1 mol-1.
The term molar absorptivity for molar absorption coefficient should
be avoided.
See absorbance, absorption
coefficient,Beer-Lambert law.
MULTIPHOTON ABSORPTION
See multiphoton process.
See also biphotonic
excitation.
MULTIPHOTON PROCESS
A process involving interaction of two or more photons with a molecular
entity.
See biphotonic process,
two-photon process.
MULTIPLICITY (Spin Multiplicity)
The number of possible orientations, calculated as 2S + 1, of the
spin angular momentum corresponding to a given total spin quantum
number (S), for the same spatial electronic wavefunction. A state
of singlet multiplicity has S = 0 and 2S+ 1 = 1. A doublet state has
S = 1/2, 2S + 1 = 2, etc. Note that when S > L (the total orbital
angular momentum quantum number) there are only 2L + 1 orientations
of total angular momentum possible.

