Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V] [W]
[X] [Y] [Z]
SACRIFICIAL ACCEPTOR
Molecular entity that acts as the electron acceptor in a photoinduced
electron transfer process and is not restored in a subsequent oxidation
process but is destroyed by irreversible chemical conversion.
SACRIFICIAL DONOR
Molecular entity that acts as the electron donor in a photoinduced
electron transfer process and is not restored in a subsequent reduction
process but is destroyed by irreversible chemical conversion.
SEMICONDUCTOR LASER
See diode laser
SCHENCK SENSITIZATION
MECHANISM
The mechanism of chemical transformation of one molecular entity caused
by photoexcitation of a sensitizer which undergoes temporary covalent
bond formation with the molecular entity.
SCINTILLATORS
Materials used for the measurement of radioactivity, by recording
the radioluminescence. They contain compounds (chromophores) which
combine a high fluorescence quantum efficiency, a short fluorescence
lifetime, and a high solubility. These compounds are employed as solutes
in aromatic liquids and polymers to form organic liquid and plastic
scintillators, respectively.
SELECTION RULE
A selection rule states whether a given transition is allowed or forbidden,
on the basis of the symmetry or spin of the wavefunctions of the initial
and final states.
SELF-ABSORPTION
Absorption of part of the fluorescence from excited molecular entities
by molecular entities of the same species in the ground state. The
mechanism operating is a radiative energy transfer.
SELF-QUENCHING
Quenching of an excited atom or molecular entity by interaction with
another atom or molecular entity of the same species in the ground
state.
See also Stern-Volmer
kinetic relationships.
SENSITIZER
See photosensitizer.
SENSITIZATION
See photosensitization.
SIMULTANEOUS
PAIR TRANSITIONS
Simultaneous electronic transitions in two coupled absorbers or emitters.
Because of the coupling, transitions which are spin-forbidden in one
of the centres might become spin allowed (spin flip).
SINGLE PHOTON COUNTING
See photon counting.
SINGLE PHOTON TIMING
See time-correlated
single photon counting.
SINGLET MOLECULAR
OXYGEN
The oxygen molecule (dioxygen), O2, in an excited singlet
state. The ground state of O2 is a triplet 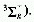 .
The two metastable singlet states derived from the ground state configuration
are
.
The two metastable singlet states derived from the ground state configuration
are 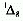 and
and 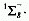 .
The term singlet oxygen alone, without mention of the chemical species
is discouraged since it can also refer to an oxygen atom in a 1S
or 1D excited state. While the oxygen atom ground state
is a triplet 3P state, the 1S and 1D
states are also derived from the ground state configuration.
.
The term singlet oxygen alone, without mention of the chemical species
is discouraged since it can also refer to an oxygen atom in a 1S
or 1D excited state. While the oxygen atom ground state
is a triplet 3P state, the 1S and 1D
states are also derived from the ground state configuration.
SINGLET-SINGLET
ANNIHILATION
See annihilation, spin
conservation rule.
SINGLET-SINGLET
ENERGY TRANSFER
Transfer of excitation from an electronically excited donor in a singlet
state to produce an electronically excited acceptor in a singlet state.
See electron
exchange excitation transfer, Förster
excitation transfer, radiative
energy transfer.
SINGLET STATE
A state having a total electron spin quantum number equal to 0.
See multiplicity.
SINGLET-TRIPLET
ENERGY TRANSFER
Transfer of excitation from an electronically excited donor in a singlet
state to produce an electronically excited acceptor in a triplet state.
See spin
conservation rule.
SOLAR CONVERSION
EFFICIENCY
The ratio of the Gibbs energy gain per unit time per m2
of surface exposed to the sun to and the solar irradiance, E., integrated
between  = 0 and
= 0 and  =
=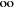 .
.
SOLID
STATE LASERS
CW or pulsed lasers in which the active medium is a solid matrix (crystal
or glass) doped with an ion (e. g., Nd3+, Cr3+,
Er3+). The emitted wavelength depends on the active ion,
the selected optical transition, and the matrix. Some of these lasers
are tunable within a very broad range (e. g., from 700 to 1000 nm
for Ti3+ doped sapphire). Pulsed lasers may be free-running,
Q-switched, or mode-locked. Some CW lasers may be mode-locked.
SOLVATOCHROMISM
The (pronounced) change in position of an electronic absorption or
emission band, accompanying a change in solvent polarity.
SOLVENT-SEPARATED
ION PAIR
Pair of ions separated by at least one solvent molecule. During electron-transfer
processes between neutral molecular species, solvent separated ion
pairs may form either directly or via solvation-induced separation
of contact ion pairs.
See also contact ion
pair.
SOLVENT SHIFT
A shift in the frequency of a spectral band of a chemical species
arising from interaction with its solvent environment.
See bathochromic
shift, hypsochromic shift,
solvatochromism
SONOLUMINESCENCE
Luminescence induced by sound waves.
See triboluminescence.
SPECIFIC PHOTON EMISSION
Same as photon exitance.
SPECTRAL (PHOTON) EFFECTIVENESS
The reciprocal of the photon fluence rate, 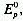 ,
at wavelength
,
at wavelength  ,
causing identical photoresponse,
,
causing identical photoresponse, 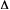 y,
per unit time (
y,
per unit time (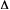 y/
y/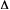 t).
The effectiveness spectrum is directly proportional to the conversion
spectrum of the sensory pigment, if spectral attenuance is negligible.
t).
The effectiveness spectrum is directly proportional to the conversion
spectrum of the sensory pigment, if spectral attenuance is negligible.
SPECTRAL IRRADIANCE (E )
)
Irradiance, E, at wavelength  per unit wavelength interval. The SI unit is Wm-3, but
a commonly used unit is W m-3 nm-1.
per unit wavelength interval. The SI unit is Wm-3, but
a commonly used unit is W m-3 nm-1.
SPECTRAL OVERLAP
In the context of radiative energy transfer, it is the integral, 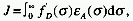 ,
which measures the overlap of the emission spectrum of the excited
donor, D, and the absorption spectrum of the ground state acceptor,
A. f 'D is the measured normalized emission of D,
,
which measures the overlap of the emission spectrum of the excited
donor, D, and the absorption spectrum of the ground state acceptor,
A. f 'D is the measured normalized emission of D,
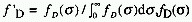 is the photon exitance of
the donor at wavenumber
is the photon exitance of
the donor at wavenumber 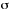 ,
and
,
and 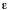 A
(
A
(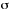 )
is the decadic molar absorption coefficient of A at wavenumber
)
is the decadic molar absorption coefficient of A at wavenumber 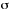 .
.
In the context of Förster excitation transfer
J is given by:
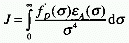
In the context of Dexter excitation transfer J is given
by
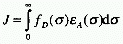
In this case fD and 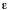 A,
the emission spectrum of donor and absorption spectrum of acceptor,
respectively, are both normalized to unity, so that the rate constant
for energy transfer, kET , is independent of the oscillator
strength of both transitions (contrast to Förster mechanism).
For the units of J, see the list of symbols.
A,
the emission spectrum of donor and absorption spectrum of acceptor,
respectively, are both normalized to unity, so that the rate constant
for energy transfer, kET , is independent of the oscillator
strength of both transitions (contrast to Förster mechanism).
For the units of J, see the list of symbols.
See energy transfer.
SPECTRAL PHOTON EXITANCE
(Mp )
)
The photon exitance, Mp, at wavelength  per unit wavelength interval. The SI unit is s-1 m-3,
but a commonly used unit is s-1 m-2 nm-1.
Alternatively, the term can be used with the amount of photons (mol
or its equivalent einstein), the SI unit then being mol s-1
m-3 and the common unit mol s-1 m-2
nm-1.
per unit wavelength interval. The SI unit is s-1 m-3,
but a commonly used unit is s-1 m-2 nm-1.
Alternatively, the term can be used with the amount of photons (mol
or its equivalent einstein), the SI unit then being mol s-1
m-3 and the common unit mol s-1 m-2
nm-1.
SPECTRAL PHOTON FLOW
(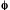 p
p )
)
The photon flow, 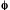 per unit wavelength interval. The SI unit is s-1 m-1,
but a commonly used unit is s-1 nm-1. Alternatively,
the term can be used with the amount of photons (mol or its equivalent
einstein), the SI unit then being mol s-1 m-1
and the common unit mol s-1 nm-1.
per unit wavelength interval. The SI unit is s-1 m-1,
but a commonly used unit is s-1 nm-1. Alternatively,
the term can be used with the amount of photons (mol or its equivalent
einstein), the SI unit then being mol s-1 m-1
and the common unit mol s-1 nm-1.
SPECTRAL
PHOTON FLUX (PHOTON IRRADIANCE) (Ep )
)
The photon irradiance, Ep, at wavelength  per unit wavelength interval. The SI unit is s-1 m-3,
but a commonly used unit is s-1 m-2 nm-1.
Alternatively, the term can be used with the amount of photons (mol
or its equivalent einstein), the SI unit then being mol s-1
m-3 and the common unit mol s-1 m-2
nm-1.
per unit wavelength interval. The SI unit is s-1 m-3,
but a commonly used unit is s-1 m-2 nm-1.
Alternatively, the term can be used with the amount of photons (mol
or its equivalent einstein), the SI unit then being mol s-1
m-3 and the common unit mol s-1 m-2
nm-1.
SPECTRAL PHOTON RADIANCE
(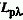 )
)
The photon radiance, Lp, at wavelength  per unit wavelength interval. The SI unit is s-1 m-3
sr-1, but a commonly used unit is s-1 m-2
sr-1 nm-1. Alternatively, the term can be used
with the amount of photons (mol or its equivalent einstein), the SI
unit then being mol s-1 m-3 sr-1
and the common unit mol s-1 m-2sr-1
nm-1.
per unit wavelength interval. The SI unit is s-1 m-3
sr-1, but a commonly used unit is s-1 m-2
sr-1 nm-1. Alternatively, the term can be used
with the amount of photons (mol or its equivalent einstein), the SI
unit then being mol s-1 m-3 sr-1
and the common unit mol s-1 m-2sr-1
nm-1.
SPECTRAL RADIANCE (L )
)
The radiance, L, at wavelength  per unit wavelength interval. The SI unit is W m-3 sr-1,
but a commonly used unit is W m-2 sr-1 nm-1.
per unit wavelength interval. The SI unit is W m-3 sr-1,
but a commonly used unit is W m-2 sr-1 nm-1.
SPECTRAL RADIANT
EXITANCE (M )
)
The radiant exitance, M, at wavelength  per unit wavelength interval. The SI unit is W m-3, but
a commonly used unit is W m-2 nm-1.
per unit wavelength interval. The SI unit is W m-3, but
a commonly used unit is W m-2 nm-1.
SPECTRAL RADIANT FLUX
Same as spectral radiant power.
SPECTRAL RADIANT
INTENSITY (I )
)
The radiant intensity, I, at wavelength  per unit wavelength interval. The SI unit is W m-1 sr-1,
but a commonly used unit is W nm-1 sr-1.
per unit wavelength interval. The SI unit is W m-1 sr-1,
but a commonly used unit is W nm-1 sr-1.
SPECTRAL RADIANT POWER
(P )
)
The radiant power at wavelength  per unit wavelength interval. The SI unit is W m-1, but
a commonly used unit is W nm-1.
per unit wavelength interval. The SI unit is W m-1, but
a commonly used unit is W nm-1.
SPECTRAL RESPONSIVITY
The spectral output quantity of a system such as a photomultiplier,
diode array, photoimaging device, or biological unit divided by the
spectral irradiance s( )
= dy(
)
= dy( )/dE(
)/dE( ),
simplified expression: s(
),
simplified expression: s( )
= Y
)
= Y /E
/E
 ,
where Y
,
where Y is the magnitude of the output signal for irradiation at wavelength
is the magnitude of the output signal for irradiation at wavelength
 and E
and E is the spectral irradiance of parallel and perpendicular incident
beam at the same wavelength.
is the spectral irradiance of parallel and perpendicular incident
beam at the same wavelength.
SPECTRAL SENSITIVITY
See spectral responsivity.
SPECTRAL SENSITIZATION
The process of increasing the spectral responsivity of a (photoimaging)
system in a certain wavelength region.
SPHERICAL RADIANCE
Same as radiant exitance, M. It is the integration of the radiant
power, P, leaving a source over the solid angle and over the whole
wavelength range. The SI unit is W m-2.
SPHERICAL RADIANT
EXPOSURE
Same as fluence.
SPIN-ALLOWED
ELECTRONIC TRANSITION
An electronic transition which does not involve a change in the spin
part of the wavefunction.
SPIN CONSERVATION RULE
(Wigner rule)
Upon transfer of electronic energy between an excited atom or molecular
entity and other atom or molecular entity in its ground or excited
state, the overall spin angular momentum of the system, a vector quantity,
should not change.
See annihilation.
SPIN FLIP
See simultaneous pair
transitions.
SPIN-ORBIT COUPLING
The interaction of the electron spin magnetic moment with the magnetic
moment due to the orbital motion of the electron. One consequence
of spin-orbit coupling is the mixing of zero-order states of different
multiplicity. This effect may result in fine structure called spin-orbit
splitting.
SPIN-ORBIT SPLITTING
Removal of state degeneracy by spin-orbit coupling.
SPIN-SPIN COUPLING
The interaction between the spin magnetic moments of different electrons
and/or nuclei. It causes, e.g. the multiplet pattern in nuclear magnetic
resonance spectra.
SPONTANEOUS EMISSION
That mode of emission which occurs even in the absence of a perturbing
external electromagnetic field. The transition between states, n and
m, is governed by the Einstein coefficient of spontaneous emission,
Anm.
See also stimulated
emission.
STARK EFFECT
Splitting or shifts of spectral lines in an electric field. Also called
electrochromic effect.
STATE CROSSING
See avoided crossing, surface
crossing.
STATE DIAGRAM
See Jablonski diagram.
STATIC QUENCHING
See quenching.
STERN-VOLMER
KINETIC RELATIONSHIPS
This term applies broadly to variations of quantum yields of photophysical
processes (e.g., fluorescence or phosphorescence) or photochemical
reaction (usually reaction quantum yield) with the concentration of
a given reagent which may be a substrate or a quencher. In the simplest
case, a plot of 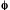 0/
0/
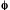 (or M0/M for emission) vs. concentration of quencher, [Q],
is linear, obeying the equation
(or M0/M for emission) vs. concentration of quencher, [Q],
is linear, obeying the equation
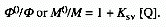 (1)
(1)
Inequation (1) Ksv is referred to as the
Stern-Volmer constant. Equation (1) applies when a quencher inhibits
either a photochemical reaction or a photophysical process by a single
reaction.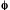 0
and M0 are the quantum yield and emission intensity (radiant
exitance), respectively, in the absence of the quencher Q, while
0
and M0 are the quantum yield and emission intensity (radiant
exitance), respectively, in the absence of the quencher Q, while 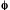 and M are the same quantities in the presence of the different concentrations
of Q. In the case of dynamic quenching the constant Ksv
is the product of the true quenching constant kq and the
excited state lifetime,
and M are the same quantities in the presence of the different concentrations
of Q. In the case of dynamic quenching the constant Ksv
is the product of the true quenching constant kq and the
excited state lifetime,  0,
in the absence of quencher. kq is the bimolecular reaction
rate constant for the elementary reaction of the excited state with
the particular quencher Q. Equation (1) can therefore be replaced
by the expression (2)
0,
in the absence of quencher. kq is the bimolecular reaction
rate constant for the elementary reaction of the excited state with
the particular quencher Q. Equation (1) can therefore be replaced
by the expression (2)
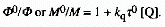 (2)
(2)
When an excited state undergoes a bimolecular reaction
with rate constant kr to form a product, a double-reciprocal
relationship is observed according to the equation
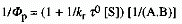 (3)
(3)
where 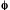 p
is the quantum efficiency of product formation, A the efficiency of
forming the reactive excited state, B the fraction of reactions of
the excited state with substrate S which leads to product, and [S]
is the concentration of reactive ground-state substrate. The intercept/slope
ratio gives kr
p
is the quantum efficiency of product formation, A the efficiency of
forming the reactive excited state, B the fraction of reactions of
the excited state with substrate S which leads to product, and [S]
is the concentration of reactive ground-state substrate. The intercept/slope
ratio gives kr 0.
If [S] = [Q], and if a photophysical process is monitored, plots of
equations (2) and (3) should provide independent determinations of
the product-forming rate constant kr. When the lifetime
of an excited state is observed as a function of the concentration
of S or Q, a linear relationship should be observed according to the
equation
0.
If [S] = [Q], and if a photophysical process is monitored, plots of
equations (2) and (3) should provide independent determinations of
the product-forming rate constant kr. When the lifetime
of an excited state is observed as a function of the concentration
of S or Q, a linear relationship should be observed according to the
equation
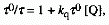 (4)
(4)
where 0
is the lifetime of the excited state in the absence of the quencher
Q.
0
is the lifetime of the excited state in the absence of the quencher
Q.
See also self-quenching.
STIMULATED EMISSION
That part of the emission which is induced by a resonant perturbing
electromagnetic field. The transition between states, n and m, is
governed by the Einstein coefficient of stimulated emission, Bnm.
CIDNP emission and lasing action are examples of processes which require
stimulated emission.
See also spontaneous
emission.
STOKES SHIFT
The difference (usually in frequency units) between the spectral positions
of the band maxima (or the band origin) of the absorption and luminescence
arising from the same electronic transition. Generally, the luminescence
occurring at a longer wavelength than the absorption is stronger than
the opposite. The latter may be called an anti-Stokes shift.
SUPEREXCHANGE INTERACTION
Electronic interaction between two molecular entities mediated by
one or more different molecules or ions.
SUPERRADIANCE
Spontaneous emission amplified by a single pass through a population
inverted medium. It is distinguished from true laser action by its
lack of coherence. The term superradiance is frequently used in laser
technology.
See coherent radiation.
SURFACE CROSSING
In a diagram of electronic energy versus molecular geometry, the electronic
energies of two states of different symmetry may be equal at certain
geometrical parameters. At this point (unidimensional representation),
line or surface (more than one dimension), the two potential-energy
surfaces are said to cross one another.
See avoided crossing.

