Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V]
[W] [X] [Y]
[Z]
LAMBERT LAW
The fraction of light absorbed by a system is independent of the incident
spectral radiant power (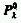 ). This
law holds only if
). This
law holds only if 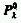 is small,
scattering is negligible, and multiphoton processes, excited state
populations, and photochemical reactions are negligible.
is small,
scattering is negligible, and multiphoton processes, excited state
populations, and photochemical reactions are negligible.
See absorbance, Beer-Lambert
law.
LAMP
A source of incoherent radiation.
See high-pressure
mercury lamp(arc), medium-pressure
mercury lamp(arc), and low-pressure
mercury lamp (arc), and antimony-xenon
lamp (arc), mercury-xenon
lamp (arc), quartz-iodine lamp,
tungsten-halogen lamp, resonance
lamp and xenon lamp.
LAPORTE RULE
For monophotonic radiative transitions in centro-symmetric systems,
the only nonvanishing electric-dipole transition moments are those
which connect an even term (g) with an odd term (u).
LASER
A source of ultraviolet, visible, or infrared radiation which produces
light amplification by stimulated emission of radiation from which
the acronym is derived. The light emitted is coherent except for superradiance
emission.
See argon ion laser,
helium-cadmium laser, chemical
laser, CO2 laser, copper
vapour laser, diode lasers, dye
laser, excimer laser, free
electron laser, free-running
laser, gas lasers, helium-neon
laser, krypton ion laser,
mode-locked laser, neodymium
laser, nitrogen laser, Q-switched
laser, solid state lasers
and ruby laser.
See also lasing.
LASING
The process of light amplification by stimulated emission of radiation
(laser).
LATENT IMAGE
The primary result of radiation absorption in a photo-imaging system
which is susceptible to development.
LIFETIME ( )
)
The lifetime of a molecular entity which decays in a first-order process
is the time needed for a concentration of the entity to decrease to
1/e of its original value. Statistically, it represents the life expectation
of the entity. It is equal to the reciprocal of the sum of the (pseudo)unimolecular
rate constants of all processes which cause the decay. Lifetime is
used sometimes for processes which are not first order. However, in
such cases, the lifetime depends on the initial concentration of the
entity, or of a quencher and therefore only an initial or a mean lifetime
can be defined. In this case it should be called apparent lifetime,
instead. Occasionally, the term half-life ( 1/2)
is used, representing the time needed for the concentration of an
entity to decrease to one half of its original value.
1/2)
is used, representing the time needed for the concentration of an
entity to decrease to one half of its original value.
LIGAND FIELD SPLITTING
The removal of a degeneracy of atomic or molecular levels in a molecule
or ion with a given symmetry induced by the attachment or removal
of ligands to produce reduced symmetries.
See crystal
field splitting.
LIGAND
TO LIGAND CHARGE TRANSFER (LLCT) TRANSITION
An electronic transition of a metal complex that corresponds to excitation
populating an electronic state in which considerable electron transfer
between two ligands has occurred.
LIGAND
TO METAL CHARGE TRANSFER (LMCT) TRANSITION
An electronic transition in a metal complex that corresponds to excitation
populating an electronic state in which considerable electron transfer
from a ligand to a metal center has occurred.
See also metal
to ligand charge transfer transition
LIGHT POLARIZATION
When the end point of the electric vector of a polarized light beam
is viewed along the direction of light propagation, it moves along
a straight line if the light is linearly polarized, along a circle
if it is circularly polarized, and along an ellipse if it is elliptically
polarized.
LIGHT SOURCE
See lamp, laser
LORENTZIAN BAND SHAPE
This band shape is described by the function

where 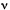 0
is the mean band position,
0
is the mean band position, 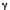 is the half band width at half maximum, and F(
is the half band width at half maximum, and F(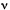 -
-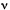 0)
is the frequency distribution function.
0)
is the frequency distribution function.
See also Gaussian
band shape.
LOW-PRESSURE
MERCURY LAMP (Arc)
A type of resonance lamp which contains mercury vapour at pressures
of about 0.1 Pa (0.75 x 10-3 Torr; 1 Torr = 133.3 Pa).
At 25 oC, such a lamp emits mainly at 253.7 and 184.9 nm.
Other terms used for such a lamp are germicidal, cold and hot cathode,
Wood lamp.
See lamp.
LUMINESCENCE
Spontaneous emission of radiation from an electronically or vibrationally
excited species not in thermal equilibrium with its environment.
See also bioluminescence,
chemiluminescence, electrogenerated
luminescence, fluorescence, phosphorescence,
photoluminescence, radioluminescence,
sono luminescence, thermoluminescence,
triboluminescence.
LUMIPHORE (Luminophore)
A part of a molecular entity (or atom or group of atoms) in which
electronic excitation associated with a given emission band is approximately
localized. (Analogous to chromophore for absorption spectra.)

