Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V]
[W] [X] [Y]
[Z]
IMAGING (Photoimaging)
The use of a photosensitive system for the capture, recording, and
retrieval of information associated with an object using electromagnetic
energy.
INCOHERENT RADIATION
Not having the properties of the coherent radiation.
INNER FILTER EFFECT
This term is used in two different ways. In an emission experiment,
it refers to an apparent decrease in emission quantum yield and/or
distortion of bandshape as a result of reabsorption of emitted radiation.
During a light irradiation experiment, absorption of incident radiation
by a species other than the intended primary absorber is also described
as an inner filter effect.
INNER-SPHERE
ELECTRON TRANSFER
Historically an electron transfer between two metal centers sharing
a ligand or atom in their respective coordination shells. The definition
has more recently been extended to any situation in which the interaction
between the donor and acceptor centers in the transition state is
significant (> 20 kJ mol-1,).
Compare outer-sphere electron transfer
INTEGRATING-SPHERE
A hollow sphere having a highly reflecting inside surface used as
a device to collect, with very high efficiency, light scattered or
emitted from a sample contained in it or located outside and near
one of the ports. Small ports allow the entrance of light and access
to a detector.
INTENDED CROSSING (of
"Potential-Energy Surfaces")
Same as avoided crossing. The term 'intended' should not be used in
this context since it is an anthropomorphic term.
INTENSITY
Traditional term for photon flux, fluence rate, irradiance or radiant
power (radiant flux). In terms of an object exposed to radiation,
the term should now be used only for qualitative descriptions.
INTENSITY (I) (of a light
source)
Same as radiant intensity.
INTENSITY
(of a spectral feature)
Describes the magnitude of the particular feature in the spectrum.
INTERFERENCE FILTER
See filter.
INTERFEROMETER
See Fourier transform
spectrometer.
INTERNAL CONVERSION
A photophysical process. Isoenergetic radiationless transition between
two electronic states of the same multiplicity. When the transition
results in a vibrationally excited molecular entity in the lower electronic
state, this usually undergoes deactivation to its lowest vibrational
level, provided the final state is not unstable to dissociation.
INTERNAL TRANSMITTANCE
See transmittance.
INTERSYSTEM CROSSING
A photophysical process. Isoenergetic radiationless transition between
two electronic states having different multiplicities. It often results
in a vibrationally excited molecular entity in the lower electronic
state, which then usually deactivates to its lowest vibrational level.
INTERVALENCE
CHARGE TRANSFER
Electron transfer (thermal or photoinduced) between two metal sites
differing only in oxidation state. Quite often such electron transfer
reverses the oxidation states of the sites. The term is frequently
extended to the case of metal-to-metal charge transfer between non-equivalent
metal centers.
INTIMATE ION PAIR
See contact ion pair
INVERTED REGION (for electron
transfer)
In plots relating rate constants to changes in standard Gibbs energy
(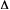 Go)
for electron transfer a region may occur in which the rate constants
decrease as the exergonicity of the reaction increases. This region
is often referred to as the inverted region and its presence is predicted
by the theory developed for outer sphere electron transfer for the
case -
Go)
for electron transfer a region may occur in which the rate constants
decrease as the exergonicity of the reaction increases. This region
is often referred to as the inverted region and its presence is predicted
by the theory developed for outer sphere electron transfer for the
case -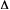 Go
>
Go
>  in the Marcus equation,
in the Marcus equation,  being the reorganization energy.
being the reorganization energy.
Note the similarity to the energy gap law for radiationless
conversion of an excited state.
Compare normal region.
IRRADIANCE (E)
The radiant flux or radiant power, P, of all wavelengths incident
on an infinitesimal element of surface containing the point under
consideration divided by the area of the element (dP/dS, simplified
expression: E = P/S when the radiant power is constant over the surface
area considered). The SI unit is W m-2. Note that 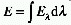 , where E
, where E is the spectral irradiance at wavelength
is the spectral irradiance at wavelength  .
For a parallel and perpendicularly incident beam not scattered or
reflected by the target or its surroundings fluence rate (E0)
is an equivalent term.
.
For a parallel and perpendicularly incident beam not scattered or
reflected by the target or its surroundings fluence rate (E0)
is an equivalent term.
See also photon irradiance,
spectral irradiance.
ISOABSORPTION POINT
The use of this term, equivalent to isosbestic point, is not recommended.
ISOCLINIC POINT
A wavelength, wavenumber, or frequency at which the first derivative
of an absorption spectrum of a sample does not change upon a chemical
reaction or physical change of the sample.
ISOEMISSIVE POINT
Same as isostilbic point.
ISOOPTOACOUSTIC POINT
A wavelength, wavenumber, or frequency at which the total energy emitted
by a sample as heat does not change upon a chemical reaction or physical
change of the sample. Its position depends on the experimental conditions.
The spectral differences between the isosbestic points and the isooptoacoustic
points are the result of the nonlinear relationship between the molar
absorption coefficient and the photoacoustic signal.
See photoacoustic
spectroscopy.
ISOSBESTIC POINT
A wavelength, wavenumber, or frequency at which the total absorbance
of a sample does not change during a chemical reaction or a physical
change of the sample.The term derives from the Greek word for 'same
attenuance'. A simple example occurs when one molecular entity is
converted into another which has the same molar absorption coefficient
at a given wavelength. As long as the sum of the concentrations of
the two molecular entities in the solution is held constant, there
will be no change in absorbance at this wavelength as the ratio of
the two entities is varied. In general, 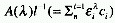 must remain constant during the reaction or physical change in order
to observe an isosbestic point. The use of the term isoabsorption
point is not recommended.
must remain constant during the reaction or physical change in order
to observe an isosbestic point. The use of the term isoabsorption
point is not recommended.
ISOSTILBIC POINT
The wavelength at which the intensity of emission of a sample does
not change during a chemical reaction or physical change. The term
derives from the Greek word for 'same luminescence'. The terms isoemissive
and isolampsic are sometimes used.
See isosbestic point.

