Glossary of Terms Used in Photochemistry
[A] [B] [C]
[D] [E] [F]
[G] [H] [I]
[J] [K] [L]
[M]
[N] [O] [P]
[Q] [R] [S]
[T] [U] [V] [W]
[X] [Y] [Z]
EFFECTIVENESS
See spectral effectiveness.
EFFICIENCY (of a step; 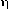 )
)
The ratio between the useful energy delivered or bound and the energy
supplied, i.e., energy output/energy input. It is also used in the
sense of a quantitative measure of the relative rate of a given step
involving a species with respect to the sum of the rates of all of
the parallel steps which depopulate that species.
See also quantum yield.
EFFICIENCY SPECTRUM
A plot of the efficiency of a step (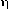 )
against wavelength or photon energy.
)
against wavelength or photon energy.
See action spectrum,
conversion spectrum.
Compare spectral effectiveness.
EINSTEIN
One mole of photons. Although widely used, it is not an IUPAC sanctioned
unit. It is sometimes defined as the energy of one mole of photons.
This use is discouraged.
ELECTROCHEMILUMINESCENCE
See electrogenerated
luminescence.
ELECTROCHROMIC EFFECT
See Stark effect.
ELECTROGENERATED
LUMINESCENCE (ECL)
Luminescence produced by electrode reactions. Also called electroluminescence
or electrochemiluminescence.
ELECTROLUMINESCENCE
See electrogenerated
luminescence.
ELECTRON CORRELATION
The adjustment of electron motion to the instantaneous (as opposed
to time-averaged) positions of all the electrons in a molecular entity.
See also correlation
energy.
ELECTRON
EXCHANGE EXCITATION TRANSFER
Same as Dexter excitation transfer.
See also energy transfer.
ELECTRONIC CONFIGURATION
See configuration.
ELECTRONIC ENERGY
MIGRATION (or Hopping)
The movement of electronic excitation energy from one molecular entity
to another of the same species, or from one part of a molecular entity
to another of the same kind (e.g. excitation migration between the
chromophores of an aromatic polymer). The migration can happen via
radiative or radiationless processes.
ELECTRONICALLY
EXCITED STATE
A state of an atom or molecular entity which has greater electronic
energy than the ground state of the same entity.
ELECTRON TRANSFER
The transfer of an electron from one molecular entity to another or
between two localized sites in the same molecular entity.
See also inner-sphere
electron transfer, outer-sphere
electron transfer, Marcus equation.
ELECTRON
TRANSFER PHOTOSENSITIZATION
Photochemical process in which a reaction of a non-absorbing substrate
is induced by electron transfer (not energy transfer) with an excited
light-absorbing sensitizer. The overall process must be such that
the sensitizer is recycled. Depending on the action of the excited
sensitizer as electron donor or acceptor the sensitization is called
reductive or oxidative.
See also photosensitization.
ELECTROPHOTOGRAPHY
Processes of photoimaging which are based on photo-induced changes
of electric fields (photo-conductive or photo-electrostatic effects).
EL-SAYED RULES
The rate of intersystem crossing, e.g.from the lowest singlet state
to the triplet manifold, is relatively large if the radiationless
transition involves a change of orbital type.E.g. 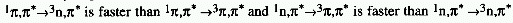
See multiplicity.
EMISSION
Radiative deactivation of an excited state; transfer of energy from
a molecular entity to an electromagnetic field.
See also fluorescence
, luminescence, phosphorescence.
EMISSION SPECTRUM
Plot of the emitted spectral radiant power (spectral radiant exitance)
or of the emitted spectral photon irradiance (spectral photon exitance)
against a quantity related to photon energy, such as frequency, 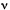 ,
wavenumber,
,
wavenumber, 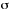 ,
or wavelength,
,
or wavelength, .
When corrected for wavelength dependent variations in the equipment
response, it is called a corrected emission spectrum.
.
When corrected for wavelength dependent variations in the equipment
response, it is called a corrected emission spectrum.
EMITTANCE
See radiant exitance.
ENCOUNTER COMPLEX
An intermolecular ensemble formed by molecular entities in contact
or separated by a distance small compared to the diameter of solvent
molecules and surrounded by several shells of solvent molecules; the
innermost shell is the solvent "cage". If one of the species is excited,
the excitation usually takes place prior to formation of the encounter
complex. During the lifetime of the encounter complex the reactants
can collide several times to form collision complexes, and then undergo
structural and electronic changes. If the interaction between the
reactants leads to a minimum in the potential energy and one of the
entities is electronically excited, the encounter complex may represent
an exciplex or excimer.
See also contact ion
pair and collision complex.
ENERGY MIGRATION
See electronic energy migration.
ENERGY POOLING
See annihilation.
ENERGY STORAGE EFFICIENCY
(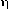 )
)
The rate of the Gibbs energy storage in an endothermic photochemical
reaction divided by the incident irradiance.
See also efficiency.
ENERGY TRANSFER
From a phenomenological point of view, the term is used to describe
the process by which a molecular entity absorbs light and a phenomenon
originates from the excited state of another molecular entity. In
mechanistic photochemistry the term has been reserved for the photophysical
process in which an excited state of one molecular entity (the donor)
is deactivated to a lower-lying state by transferring energy to a
second molecular entity (the acceptor), which is thereby raised to
a higher energy state. The excitation may be electronic, vibrational,
rotational or translational. The donor and acceptor may be two parts
of the same molecular entity, in which case the process is called
intramolecular energy transfer.
See also Dexter
excitation transfer, Förster
excitation transfer, radiative
energy transfer, and spectral
overlap.
ENERGY TRANSFER PLOT
A plot of the quenching rate constant of an excited molecular entity
by a series of quenchers versus the excited state energy of the quenchers.
Alternatively, a plot of the rate constant for the sensitization of
a reaction versus the excited state energy of different sensitizers.
This type of plot is used to estimate the energy of the excited molecular
entity quenched (in the former case) or produced (in the latter case).
Also known as Hammond-Herkstroeter plot.
See also Stern-Volmer
kinetic relationships.
ENHANCER
A fluorescent compound which accepts energy and thus enhances or promotes
the emission from a sample containing a chemically or enzymatically
generated excited molecular entity.
ESCA
See photoelectron spectroscopy.
EXCIMER
An electronically excited dimer, "nonbonding" in the ground state,
a complex formed by the interaction of an excited molecular entity
with a ground state partner of the same structure.
See also exciplex.
EXCIMER LASER
A source of pulsed coherent radiation obtained from an exciplex. The
proper name should be exciplex laser. Typical lasing species are noble
gas halides (XeCl, KrF, etc.) emitting in the UV domain.
See laser, gas
lasers.
EXCIPLEX
An electronically excited complex, of definite stoichiometry, "nonbonding"
in the ground state. For example, a complex formed by the interaction
of an excited molecular entity with a ground state partner of a different
structure.
If the partners have pronounced electron-donor and -acceptor character
their exciplex attains ion-pair character. The terms compact exciplex
and loose exciplex have sometimes been used to indicate that such
polar exciplexes may have structures closely related to a contact
ion pair or a solvent-separated ion pair.
EXCITATION SPECTRUM
Plot of the spectral radiant exitance or of the spectral photon exitance
against the frequency (or wavenumber, or wavelength) of excitation.
When corrected for wavelength dependent variations in the excitation
radiant power this is called a corrected excitation spectrum.
See also emission
spectrum.
EXCITATION TRANSFER
Same as energy transfer.
EXCITED STATE
A state of higher energy than the ground state of a chemical entity.
In photochemistry an electronically excited state is usually meant.
EXCITON
In some applications it is useful to consider electronic excitation
as if a quasi-particle capable of migrating, were involved. In organic
materials two models are used: the band or wave model (low temperature,
high crystalline order) and the hopping model (higher temperature,
low crystalline order or amorphous state). Energy transfer in the
hopping limit is identical with energy migration.
See electronic
energy migration.
EXITANCE
See radiant exitance.
EXTERNAL HEAVY
ATOM EFFECT
See heavy atom effect.
EXTERPLEX
Termolecular analogue of an exciplex. Use of this term is discouraged.
See also : exciplex
EXTINCTION
This term, equivalent to absorbance, is no longer recommended.
EXTINCTION COEFFICIENT
This term, equivalent to molar (decadic) absorption coefficient, is
no longer recommended.
See Beer-Lambert law.

