Glossary of terms used in theoretical
organic chemistry
[A] [B]
[C] [D] [E]
[F] [G] [H]
[I] [J-K] [L]
[M]
[N] [O] [P]
[Q-R] [S] [T]
[U-V] [W-Z]
T
Thermochemical resonance
energy - see Resonance
energy, various types of.
Thermodynamic stability -
Those species are considered to be thermodynamically stable whose
free energies are sufficiently low compared to all reasonable decomposition
products so that detectable amounts of the former may exist in equilibrium.
Three-center, four-electron
(3c-4e) bond - see Hypervalency.
Three-center, two-electron
(3c - 2e) bond - A kind of multicenter
s-bond in which three atomic cores are
held together by two electrons. The 3c-2e bonds are particularly common
in the boron hydrides, carbocations (for example, the bond in the
CH2 fragment of the methanium cation, see Hypercoordination),
bridged metal alkyls and metal halides. The simplest example of the
3c-2e bond is in the H3+ ion.
Through-bond interaction
- An intramolecular orbital
interaction of spatially separated orbitals,
where the orbitals interact through their mutual mixing with
s-orbitals of the intervening framework.
Through-space interaction
- The orbital interaction
that results from direct spatial overlap of two orbitals.
Tight binding method - Approximation
of crystal orbitals in solids
by a Linear Combination of Atomic
Orbitals. In general, tight binding is approximation in quantum
chemical methods by retaining resonance
integrals only for neighboring atoms.
Topochemical principle -
Reactions in the crystalline phase proceed under minimal atomic and
molecular displacement.
Topological distance matrix -
see Topological index.
Topological electron distribution
theory - The theory of molecular structure based on the analysis
of the topological properties of scalar fields of the electron
density function r(r) and
the Laplacian of
the electron density
[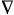 2r(r)],
which are summarized in terms of their critical points, rc,
i.e. the points where
2r(r)],
which are summarized in terms of their critical points, rc,
i.e. the points where 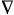 r(r)
and (-
r(r)
and (-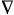 2r(r))
are equal to zero. The critical points are classified according to their
rank (number of non-zero eigenvalues) and signature (algebraic sum of
their signs) of the Hessian matrix
of r(r). Thus, a (3, -3) critical
point corresponds to a maximum of r(r)
related to an atomic nucleus, whereas a (3, -1) critical point (bond
point) is a saddle point that exists between every pair of neighboring
bonded atoms. BADER (1990); BADER,
POPELIER, and KEITH (1994).
2r(r))
are equal to zero. The critical points are classified according to their
rank (number of non-zero eigenvalues) and signature (algebraic sum of
their signs) of the Hessian matrix
of r(r). Thus, a (3, -3) critical
point corresponds to a maximum of r(r)
related to an atomic nucleus, whereas a (3, -1) critical point (bond
point) is a saddle point that exists between every pair of neighboring
bonded atoms. BADER (1990); BADER,
POPELIER, and KEITH (1994).
See also Atoms in molecules,
theory of .
Topological index - A numerical
value associated with chemical constitution purporting for correlation
of chemical structure with various physical properties, chemical
reactivity or biological activity. The numerical basis for topological
indices is provided (depending on how a molecular
graph is converted into a numerical value) by either the
adjacency matrix or the
topological distance matrix.
In the latter the topological distance between two verices is the number
of edges in the shortest path between these.
Topological resonance energy
(TRE) - see Resonance
energy, various types of.
Topomerization - see
Automerization.
Total energy of a molecular system -
The sum of the total electronic energy, Eee and
the energy of internuclear repulsion, Enr. In the
Hartree-Fock (SCF) method,
the value of Eee for a closed-shell
molecular system is given by
Eee = 2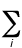 ei +
ei + 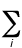
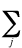 (2Jij - Kij)
(2Jij - Kij)
where ei stands for
orbital energies and Jij and Kij
stand for respectively Coulomb
and exchange integrals,
the summation being done over all occupied molecular
orbitals. The repulsion between the nuclei (A, B ...) is
defined by the expression:
Enr = 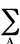
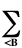 ZAZB/RAB
ZAZB/RAB
Transition state (TS) (also
transition state structure, transition structure) - The structure
corresponding to the highest point on the minimum
energy reaction path, i.e. first order saddle
point on the potential
energy surface. In transition
state spectroscopy, the definition of the transition state
is broader. It embraces the entire process of bond breaking and
bond making and is used to denote the full family of configurations
through which the reacting particles evolve along the route from
reagents to products.
See also Hessian matrix.
Transition State Theory (TST)
- The theory which provides a conceptual framework for understanding
all chemical reactivity. It serves as a powerful computational tool
for translating molecular structures and energetics into prediction
of chemical reaction rates. EYRING (1935),
GARRET and TRUHLAR (1998).
Transition state spectroscopy (TSS)
- The femtosecond temporal resolution (10-14 - 10-13
s = 10 - 100 fs) laser spectroscopy that enables one to detect the intermediate
configurations (transition states)
of a reacting system in real time and follow the nuclear motion and
energy redistribution. POLANYI and ZEWAIL
(1995).
Transition vector - The
eigenvector associated with the only negative eigenvalue of the Hessian
matrix representing the single direction in which the
potential energy surface is
a maximum. The transition vector is also a path of the intrinsic
reaction coordinate through the saddle
point.
Transmission factor - In the
equation of the theory of absolute reaction rates that relates the reaction
constant k with the height of the energy barrier
DETS, the value of the coefficient g
is equal to 1 when the conversion of reactants to products always
occurs upon attaining the height of energy barrier, and less than
1 when this condition is not met.
k = gt (kBT/h)
(QTS/QR) exp(-DETS/RT)
where k is the rate constant for the reaction of conversion
of reactants R into products by proceeding through transition state
TS; Q are partition functions per unit volume, and
t is the quantum mechanical tunnelling
coefficient. In some descriptions k = gt
is referred to as the transmission factor.
Tunnelling effect - The passage
of a particle through a potential energy barrier the height of which
is larger than the energy of that particle. The effect is important
for some processes involving the transfer of electrons and light
atoms, particularly hydrogen atoms.

