Glossary of terms used in theoretical
organic chemistry
[A] [B]
[C] [D] [E]
[F] [G] [H]
[I] [J-K] [L]
[M]
[N] [O] [P]
[Q-R] [S] [T]
[U-V] [W-Z]
I
Imaginary frequency - The
frequency of the normal vibration related to a negative eigenvalue (force
constant) of the Hessian matrix.
Transition state structure
possesses only a single imaginary frequency.
Individual Gauge
for Localized Orbitals (IGLO) - A method of calculation of
nuclear shieldings, in which localized
molecular orbitals associated with inner shell, bonding orbitals,
and lone pairs have unique origins for the calculation of diamagnetic
and paramagnetic terms. With this method, satisfactory estimates
of NMR chemical shifts for elements in the first and second rows
can be achieved in ab initio calculations with basis
sets of moderate size provided sufficiently accurate molecular
geometries are used. KUTZELNIGG (1980);
SCHINDLER and KUTZELNIGG (1983).
Inductive effect - The polarization
of chemical bonds due to shifts of their electron pairs in the direction
of the electronegative group. Within a molecule, the inductive effect
is transmitted through space and is determined by electrostatic
forces between the interacting sites. Another original (G. Lewis)
model of inductive effect relates to through-bond transmission by successive
polarization of the bonds between a dipolar or charged substituent
and the reaction site. A quantitative assessment of the inductive
effects of groups (substituents) is given by the Taft s*-constants
(for aliphatic) and the inductive sI-constants
(for aromatic) compounds.
Instability of Hartree-Fock
solution - Existence of additional solutions to the equations of
the Hartree-Fock method
occurring usually in the case when potential
energy surfaces of different electronic
states are drawn close together. Within the spin-restricted
Hartree- Fock method
(RHF), singlet and triplet instabilities are distinguished.
The former involves the existence of another solution with lower
energy and the electron distribution of lower symmetry. It may be
regarded as an indication that the initially assumed molecular shape
needs correction. The triplet instability involves rejection of
the condition of double occupancy of molecular
orbitals and thus transition to the spin-unrestricted
Hartree-Fock method (UHF).
The triplet instability is a necessary, but insufficient, condition
for the conclusion as to the biradical
character of the ground state
of a given system. MESTECHKIN (1990);
YAMAGUCHI (1981).
Internal conversion - Isoenergetic
radiationless transition
between two electronic states
of the same multiplicity.
Internal rotation - The interconversion
of stereoisomers through rotation of groups of atoms about a single
bond. The internal rotation is called "free" when the energy barrier
is so low that different rotational isomers are not perceptible
as individual chemical species (i.e. the characteristic time of the
method of observation is longer than the lifetime of the rotational
isomers). The inhibition of internal rotation by a sufficiently
high rotational barrier makes the phenomenon observable on the time
scale of the experiment, and is termed restricted or hindered rotation.
Intersystem crossing - Isoenergetic
radiationless transition
between two electronic states
of different multiplicity.
Intrinsic barrier - The activation
free-energy barrier associated with a reaction whose free-energy change
is zero.
Intrinsic reaction coordinate -
A minimum-energy reaction path
on a potential energy surface
in mass-weighted coordinates, connecting reactants to products via
the transition state.
FUKUI (1981).
Ionic bond - The bond
between atoms with sharply different electronegativities.
In strict terms, an ionic bond refers to the electrostatic attraction
experienced between the electric charges of a cation and an anion,
in contrast with a purely covalent
bond. In practice, it is preferable to consider the amount
of ionic character of a bond rather than referring to purely ionic or
purely covalent bonds. The relationship was proposed (L.Pauling)
for the estimation of ionic character of a bond between atoms A
and B:
Amount of ionic character = 1 - e-1/4 (cA
-cB)
where cA and cB
are the Pauling electronegativities of atoms A and B. This type
of bonding is realized mostly in solids.
Ionization potential of an atom
or a molecule - The minimal energy (Ix)
needed for the detachment of an electron, i.e. X + Ix
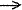 X+
+ e- . When the ion is produced in its most stable state,
(which is the case in e.g. photoionization or photoelectron spectroscopic
techniques), the values Ix correspond to adiabatic
ionization potentials. When a technique such as fast electron
bombardment is used, where the ionization occurs during the period
of collision, so that the ion X+ retains the geometry
of the initial X, the value of IX is referred to as
the vertical ionization potential.
X+
+ e- . When the ion is produced in its most stable state,
(which is the case in e.g. photoionization or photoelectron spectroscopic
techniques), the values Ix correspond to adiabatic
ionization potentials. When a technique such as fast electron
bombardment is used, where the ionization occurs during the period
of collision, so that the ion X+ retains the geometry
of the initial X, the value of IX is referred to as
the vertical ionization potential.
See also Koopmans’ theorem
Isoconjugate systems -
Molecular entities with similar conjugate networks which have the
same number of p-electrons, e.g. cyclopentadienide
and pyrrole or benzene and pyridine.
Isodesmic reaction - A reaction
(sometimes hypothetical) in which the number of bonds of each given
formal type, e.g. C-H, C-C, C=C, is conserved, but the relationship
among the bonds is altered. An example is given for a formally conceivable
reaction
F3C-C(=O)H + CH4 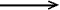 CH2F2 + H3C-C(=O)F
CH2F2 + H3C-C(=O)F
where three C-F, one C=O, and C-C and five C-H bonds are present
in both reactants and products; only the environment in which bonds
are located has changed. Due to the conservation of the number of electron
pairs in the reactants and products the changes in their correlation
energies are usually small, so that the energies of isodesmic
reactions are generally well reproduced even by simple computational
methods. This makes isodesmic reactions important interpretive tools
and a means of providing thermochemical data. GEORGE,
BOCK, and TRACHTMAN (1988); HEHRE, DITCHFIELD,
RADOM, and POPLE (1970).
Isogyric reaction - A reaction
in which the number of electron pairs is conserved, for example
CH4(1A1) + H(2S)
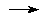 CH3(2A2")
+ H2(1S+g)
CH3(2A2")
+ H2(1S+g)
HEHRE, RADOM, SCHLEYER, and POPLE (1986).
Isolobal analogy - The
concept allowing one to establish relationships between the isolobal
groups of organic, main-group and transition metal inorganic
and organometallic compounds. The utility of the isolobal analogy
is that, if an MLn fragment is isolobal with a particular AL'm
arrangement, then one should be able to replace the former in a
molecule with the latter to form a new compound possessing a very
similar valence electron shell and even displaying similar reactivity.
The isolobal analogy makes it possible to establish qualitative
correspondence between the energy and the spatial (nodal) characteristics
of orbitals of the groups formed
by the main-group and transition elements as well as to analyze in an
approximate manner the structure and reactivity of inorganic and organometallic
compounds by analogy with those of simple organic compounds. HOFFMANN
(1982).
Isolobal groups - Molecular
moieties for which the number, symmetry properties, occupation by electrons
and approximate energy of frontier
orbitals are similar. The isolobal relationship between
the groups is denoted by a double-headed arrow with a tear-drop, for
example
CH2 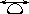 Fe(CO)4
Fe(CO)4 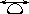 Ni(PPh3)2
Ni(PPh3)2 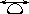 Co(h5-C5H5)CO
Co(h5-C5H5)CO
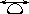 Cu(h5-C5H5)
Cu(h5-C5H5)
ALBRIGHT, BURDETT, and WHANGBO
(1985); HOFFMANN (1982).
Isostructural reaction
- A ligand exchange reaction, where the structural type of the metal
complex remains the same.
MLn + X 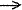 MLn-1X + L +DE
MLn-1X + L +DE
Although these types of reactions are not necessarily isodesmic
reactions, a substantial degree of error cancellation for
the calculated energy, DE, is achieved
because the coordination sphere of the metal atom M is conserved.
DAPPRICH, PIDUN, EHLERS, and FRENKING
(1995).
Isotopologues - Species that differ
only in the isotopic composition of their molecules or ions. SEEMAN,
SECOR, DISSLKAMP, and BERNSTEIN (1992).
See also isotopomers.
Isotopomers - Isomers due to the positions
of nuclear isotopes, for example CH2DOH and CH3OD.

