Glossary of terms used in theoretical
organic chemistry
[A] [B]
[C] [D] [E]
[F] [G] [H]
[I] [J-K] [L]
[M]
[N] [O] [P]
[Q-R] [S] [T]
[U-V] [W-Z]
O
Open-shell systems - Atomic
or molecular systems in which the electrons are not completely assigned
to orbitals in pairs.
Orbital (Atomic
or Molecular) - A wavefunction
which depends explicitly on the spatial coordinates of only one
electron.
Orbital correspondence analysis in maximum symmetry
(OCAMS), method of - An extension of the correlation
diagram procedure for determining reaction
paths. The reactant(s) and product(s) are set up in their
highest common symmetry point group.
The preferred reaction path retains the symmetry of a subgroup in
which all of the occupied molecular
orbitals correlate across the diagram. Inclusion of the symmetry
properties of the components of the spin-orbit
coupling operator allows application of the method to thermal
and photochemical reactions that do not conserve electron spin.
HALEVI (1992).
Orbital interaction - Interaction
of two orbitals due to their overlap which results in the formation
of two new orbitals, one of which is lower in energy than the lowest
energy level of the initial orbitals, while the energy level of
another new orbital lies higher than the highest energy level of the
initial orbitals. The notion may be extended to the interaction
of several orbitals. In perturbation
theory, the energy of the orbital interaction is proportional
to the square of the overlap integral
and inversely proportional to the energy difference between the
interacting orbitals. ALBRIGHT, BURDETT,
and WHANGBO (1985).
Orbital interaction diagram
- A diagram which portrays in a qualitative or quantitative manner the
splitting of the energy levels due to an orbital
interaction.
Orbital isomers - Species distinguishable
by different occupation of a set of available MOs. DEWAR,
KIRSHNER and KOLLMAR (1974).
Overlap integral - The integral
defining the summation over the space of the overlap of the electron
density of two orbitals:
Smn = 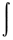 fmfndt.
fmfndt.

