Glossary of terms used in theoretical
organic chemistry
[A] [B]
[C] [D] [E]
[F] [G] [H]
[I] [J-K] [L]
[M]
[N] [O] [P]
[Q-R] [S] [T]
[U-V] [W-Z]
L
Landau-Zener model - A semiclassical
model for the probability, P, of a diabatic
reaction occurring by hopping from a potential
energy surface for one electronic
state to that of another at an avoided
crossing.
P = exp (-4p2e212
/ hV|s1 - s2|)
where e is the energy gap between the
two adiabatic electronic states at the crossing point, |s1
- s2| is the difference in slopes between the intesecting
potential curves at this point, and V is nuclear velocity
along the reaction coordinate.
SALEM (1982).
Laplacian of the electron
density - A characteristic used to reveal the distribution
of electrons in molecular systems and crystals. It is defined as
the sum of the three principal curvatures of the electron
density function at each point in space, that is
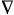 2r(r)
= �2r/�x2
+ �2r/�y2
+ �2r/�z2
2r(r)
= �2r/�x2
+ �2r/�y2
+ �2r/�z2
When 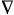 2r(r)
< 0, the value of r
at point r is greater than its average value at neighboring
points, and when
2r(r)
< 0, the value of r
at point r is greater than its average value at neighboring
points, and when 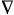 2r(r)
> 0, r at r
is less than its average value at neighboring points. Thus, the
Laplacian of the electron density identifies regions of molecular space
where r(r) is locally concentrated
or depleted; this information can be used to study directions of electrophilic
and nucleophilic attacks. A negative value of
2r(r)
> 0, r at r
is less than its average value at neighboring points. Thus, the
Laplacian of the electron density identifies regions of molecular space
where r(r) is locally concentrated
or depleted; this information can be used to study directions of electrophilic
and nucleophilic attacks. A negative value of 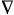 2r(r)
in the internuclear region indicates the formation of covalent
bond, while a positive value characterizes the interaction between
closed-shell molecular
(or atomic) systems. BADER
(1990).
2r(r)
in the internuclear region indicates the formation of covalent
bond, while a positive value characterizes the interaction between
closed-shell molecular
(or atomic) systems. BADER
(1990).
Least motion, principle of - The
statement that those elementary reactions are the most favored which
exhibit the fewest possible alterations in the positions of the atomic
nuclei and in the electronic
configuration. The most frequently used mathematical formulation
of the principle rests on a mechanical model of a molecule in which
the energy of structural deformation, when reactants (r) turn into
products (p), is assumed to be proportional to the sum of the squares
of the changes in the positions of the nuclei common to both reactants
and products
E = 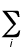 fi
(qpi - qri)2
fi
(qpi - qri)2
where fi is the force constant (in many
applications set equal to unity). The equation coincides with the
relationship for the potential energy of small vibrations, hence it
is valid only at a very early stage of a reaction. This is one of
the reasons why many reactions violate the principle of least motion.
HINE (1977).
Lennard-Jones potential
- see van der Waals
interactions.
Lewis octet rule - A classical
rule for describing the electronic
configuration of
atoms in molecules: the maximum number of electron pairs that can
be accommodated in the valence shell of a first-row element is four.
For the second and subsequent row elements there are many exceptions
to this rule. See Hypervalency.
Ligand field - see Crystal
field.
Linear Combination of Atomic Orbitals
(LCAO) - The approximation of the molecular
orbital function as a linear combination of atomic
orbitals chosen as the basis
functions.
Localized molecular orbitals (LMO)
- The molecular orbitals
located on certain fragments of a molecular system and spatially
separated from each other as much as possible. The LMOs are derived
from the electron occupied canonical
molecular orbitals by subjecting them to a unitary transformation
determined by an appropriate physical criterion, e.g. by maximizing
the sum of squares of the centroids of occupied MOs (the Foster-Boys
procedure) or by minimizing the sum of the exchange
(or Coulomb) repulsion integrals between the occupied
MOs (the Edmiston- Ruedenberg procedure). FOSTER
and BOYS (1950); EDMISTON and RUEDENBERG
(1963).
Low-spin state - When the
separation between the highest
occupied and the lowest
unoccupied molecular orbitals
is not large, two alternative electronic
states may be considered. The state with two electrons paired
up in the HOMO is called a low-spin
state. The low-spin state is the ground
state when the one-electron
energy needed to promote an electron to the LUMO is larger than
the Coulomb and exchange
repulsion energies required to pair up two electrons in
the HOMO.
See also High-spin state.
Lowest Unoccupied Molecular Orbital
(LUMO) - see Frontier
orbitals.

