Glossary of terms used in theoretical
organic chemistry
[A] [B]
[C] [D] [E]
[F] [G] [H]
[I] [J-K] [L]
[M]
[N] [O] [P]
[Q-R] [S] [T]
[U-V] [W-Z]
P
Pariser-Parr-Pople
(PPP) method - A semiempirical
quantum mechanical method of calculation of the properties
of conjugated molecules and ions from self-consistent-field
theory and the p-electron
approximation.
Pauli exclusion principle
- A rule, complementary to the aufbau
principle, of building up the electronic
configuration of atoms and molecules: a maximum of two electrons
can occupy an orbital and then
only providing that the spins of the electrons are paired, i.e. opposed.
The principle demands that the wavefunction
for a many-electron system must be asymmetric with respect to the permutation
of the space-spin coordinates for every pair of electrons.
Pauling’s bond order
- see Fractional bond number.
Peierls distortion
- The distortion of a regular one-dimensional structure with a partially
occupied band to give bond alternation, eventually leading to dimerization
or oligomerization. The degree of oligomerization l
depends on the electronic population of the conduction
band indicated by wave vector
of the Fermi level, kF:
l = 2p/kF
A Peierls distortion opens a gap at the Fermi level,
producing a net stabilization of the distorted structure. The Peierls
distortion for chain compounds is analogous to the Jahn-Teller
effect for molecules. The prototypical example of the Peierls
distortion in organic chemistry is the bond alternation present
in polyacetylene.
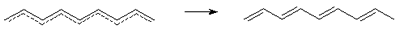
Perfect pairing -
The term used to describe corollaries of the Coulson-Rushbrooke pairing
theorem for alternant
hydrocarbons: (a) the molecular
orbitals of an even alternant hydrocarbon occur on pairs
with energies a �
Em , a
being the Coulomb integral
for carbon; (b) the coefficients at atomic
orbitals of the paired molecular orbitals are numerically the
same, but their signs at atomic orbitals of starred atoms are reversed
in going from one molecular orbital to the other; (c) in an odd
alternant hydrocarbon, with the total number of molecular orbitals odd,
all but one of the molecular orbitals is paired. The odd molecular
orbital has energy a (see nonbonding
molecular orbital) and is confined to the starred atoms.
Pericyclic reaction
- Reactions in which all first-order changes in bonding relationships
take place in concert on a closed curve, i.e. throughout a cyclic
array of continually bonded atoms forming a fully conjugated cyclic
transition state. An example
of a pericyclic reaction is the 3,3-sigmatropic (Cope) rearrangement
of hexa-1,5-diene.
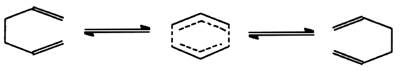
WOODWARD and HOFFMANN
(1969).
Permutation group of
the set of n objects - The set of all possible permutations that
can be applied to a given set of objects, together with the composition
operation. For example for a set of three objects (vertices of a
graph, atomic centers etc.) the permutation group consists of six permutations:
id, ((1,2)), ((1,3)), ((2,3)), ((1,2,3)), ((1,3,2)), where id is
the permutation which leaves each object fixed.
Permutational
isomerization - see Automerization.
Perturbation theory
- Along with variational method,
the second major quantum-mechanical approximation method. The methods
of perturbation theory are based on representation of the Hamiltonian
of a system under study, H, through the Hamiltonian,H0,
of a system, whose Schroedinger equation is solvable, and its relatively
small perturbation H' : H = H0+ H'. Numerous techniques are derived allowing
one to relate the unknown eigenvalues and eigenfunctions of the
perturbed system to the known eigenvalues and eigenfunctions of
the unperturbed system. As distinct from the variational method, the
methods of perturbation theory are applicable to all the electronic
states of an atom or molecule. When H' is time-dependent,
the perturbed system does not have stationary
states. In this case time-dependent perturbation theory,
which is the method of approximate calculation of the expansion of wavefunctions
of the perturbed system over wavefunctions of stationary states of the
unperturbed system must be employed. The applications of this method
are associated mostly with studies of light emission and absorption
by atoms and molecules. See also MØller-Plesset
(MP) Perturbation Theory.
Phonon - A quantized vibration
of a crystalline lattice. In contrast to photons,
phonons are quasiparticles.
Photon - The quantum of electromagnetic
energy at a given frequency.
Pi (p)
electron approximation - The assumption that in planar unsaturated
molecular entities, whose p-molecular
orbitals do not mix with s-molecular
orbitals, the molecular wavefunction
is rigorously separated into Y = YsYp
.The s-electron shell serves as a nonpolarizable
core (see core approximation)
merely providing part of the effective potential for the motion
of p-electrons, i.e. Ys
remains the same for any state of the p-system.
Point group - see
Symmetry point group.
Polarizability (static
dielectric polarizability) - A measure of the linear response of
the electronic cloud of a chemical species to a weak external electric
field. For isotropic molecules, the dipole
moment, mi, produced
by a field, E, of unit strength
mi = aE
In the general case, the polarizability is anisotropic,
i. e. dependent on the position of the molecule with respect to
the field and a is replaced by a function
(symmetrical tensor) which defines the induced dipole moment for
each possible direction of the electric field. The polarizability defined
on an experimental basis is the average polarizability, i. e. the
sum of polarizabilities (bi) in three principal
directions, b1 being collinear with the external field.
aave = (1/3) (b1
+ b2 + b3)
BÖTTCHER (1973),
EXNER (1975)
Polarization - The readjustment
of a charge distribution when it experiences an external electrostatic
potential. Polarization, p, is usually measured as the
dipole moment induced in a
unit volume
p = NdaE/M
where a is polarizability,
M/d is the molar volume (M is the molecular mass
and d is density), and E is applied electric field.
Polarized basis set
- see Basis set.
Polarizable continuum
model (PCM) - A self-consistent
reaction field method to treat solvation effects, which employs
a van der Waals surface type cavity instead of a spherical cavity in
the standard SCRF procedures.
Polyhedral skeletal
electron pair approach - see Wade’s
rules.
Potential Energy Surface
(PES) - Within the adiabatic
approximation, the function of the total energy of a molecular
system (minus kinetic energy of the nuclei) of the coordinates of
all nuclei which this system contains.
See also Energy hypersurface.
Predissociation
- Dissociation of a molecule occurring through its excitation to
a certain bound state which is coupled to a dissociative continuum.
Predissociation is caused by interaction between discrete levels
of a given electronic state
and the dissociative continuum of another electronic state. Predissociation
manifests itself by the appearance of line broadening, a decrease in
lifetime, an abrupt decrease of fluorescence quantum yield and generation
of dissociation products. KATO and BABA
(1995).
Prereactive complexes
- Weakly bound complexes with a potential minimum that precedes the
activation barrier along the reaction
path. In contrast with the van
der Waals complexes which fall apart reversibly into their constituents,
the prereactive complexes may undergo a vigorous reaction to form different
products. BÜRGER (1997)
Prochirality - The property
of an achiral object to acquire chirality
through a single desymmetrization step, e.g. addition to it of a
new atom or achiral group or substitution of an existing atom or achiral
group by different ones.
Proton affinity ( of
a given base Z) - The negative of the enthalpy change at
298K for the gas phase reaction of an atomic or molecular entity
with a proton
H+ + Z 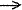 HZ+
HZ+
Pseudo Jahn-Teller
effect (synonymous with Second-order
Jahn-Teller effect) - see
Jahn- Teller effect.
Pseudopericyclic
reaction - A reaction from the subset of pericyclic
reactions for which there is no cyclic orbital overlap.
An example of pseudopericyclic orbital topology is given by the reaction
of decarbonylation of furan-2,3-dione to give 4-oxoprop-2-enal.
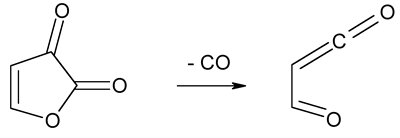
ROSS, SEIDERS, and LEMAL
(1976); BIRNEY, HAM, and UHRUH (1997).
Pseudopotential
approximation - see Core
approximation.
Pseudorotation -
A conformational change resulting in a structure that appears to
have been produced by rotation of the entire initial molecule and
is superimposable on the initial one, unless different positions
are distinguished by substitution or isotopic labeling. No angular momentum
is generated by this motion; this is the reason for the term. An
example is the facile interconversion of the envelope and twist
conformers of cyclopentane. A particular case is the Berry
pseudorotation.

