A quantitative measure of adsorption may be, and usually is, based on the following general definition: Adsorption of one or more of the components, at one or more of the phase boundaries, of a multicomponent, multiphase system, is said to occur if the concentrations in the interfacial layers are different from those in the adjoining bulk phases, so that the overall stoichiometry of the system deviates from that corresponding to a reference system of homogeneous bulk phases whose volumes and/or amounts are defined by suitably chosen dividing surfaces, or by a suitable algebraic method.
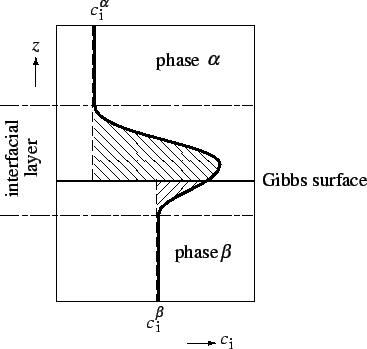
Gibbs dividing surface (or Gibbs surface10) is a geometrical surface chosen parallel to the interface defined in §1.1.2 and used to define the volumes of the bulk phases in applying the foregoing definition to the calculation of the extent of adsorption, and of other surface excess properties.
For flat or only slightly curved surfaces one is free to define the position of the Gibbs surface in the manner most convenient for the discussion of a particular problem. In what follows it is assumed that this freedom exists; it must be remembered, however, that for surfaces whose radii of curvature approach molecular dimensions, the definitions become ambiguous.
Excess thermodynamic quantities referred to the Gibbs surface are denoted by superscript ![]() to distinguish them from quantities relating to the interfacial layer, for which superscript
to distinguish them from quantities relating to the interfacial layer, for which superscript ![]() is employed.
is employed.
The surface excess amount or Gibbs adsorption of component ![]() ,
,
![]() which may be positive or negative, is defined as the excess of the amount of this component actually present in the system over that present in a reference system of the same volume as the real system and in which the bulk concentrations in the two phases remain uniform up to the Gibbs dividing surface (see Figure 1).
which may be positive or negative, is defined as the excess of the amount of this component actually present in the system over that present in a reference system of the same volume as the real system and in which the bulk concentrations in the two phases remain uniform up to the Gibbs dividing surface (see Figure 1).
|
That is
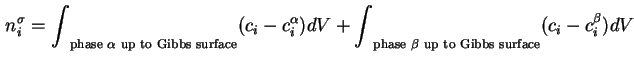
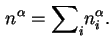
If the area (![]() ) of the interface is known, the Gibbs surface concentration or the surface excess concentration (formerly called the superficial density),
) of the interface is known, the Gibbs surface concentration or the surface excess concentration (formerly called the superficial density),
![]() , is given by
, is given by
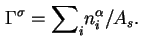
Corresponding definitions can be given for the surface excess number of molecules,
![]() , and the surface excess mass of component
, and the surface excess mass of component ![]() ,
,
![]() and for the related surface excess molecular concentration and surface excess mass concentration.
and for the related surface excess molecular concentration and surface excess mass concentration.
The detailed application of the Gibbs definition of adsorption to interfaces of different kinds is discussed in the following sections (§§1.1.9; 1.1.10; 1.1.11).