In general, the choice of the position of a Gibbs surface is arbitrary but it is possible to define quantities which are invariant with respect to this choice.
This is particularly useful for fluid/fluid interfaces where no experimental procedure exists for the unambiguous definition of a dividing surface.
The relative adsorption (
![]() or
or
![]() ). If
). If
![]() and
and
![]() are the Gibbs surface concentrations of components
are the Gibbs surface concentrations of components ![]() and 1, respectively, with reference to the same, but arbitrarily chosen, Gibbs surface, then the relative adsorption of component
and 1, respectively, with reference to the same, but arbitrarily chosen, Gibbs surface, then the relative adsorption of component ![]() with respect to component 1, defined as
with respect to component 1, defined as
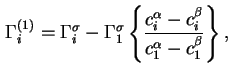
Alternatively,
![]() may be regarded as the Gibbs surface concentration of
may be regarded as the Gibbs surface concentration of ![]() when the Gibbs surface is chosen so that
when the Gibbs surface is chosen so that
![]() is zero, i.e. the Gibbs surface is chosen so that the reference system contains the same amount of component 1 as the real system. Hence
is zero, i.e. the Gibbs surface is chosen so that the reference system contains the same amount of component 1 as the real system. Hence
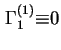 .
.
In terms of experimental quantities
![$\displaystyle {\Gamma}_i^{(1)} =A_s^{-1}[n_i - V^{\alpha}c^{\alpha}_i- V^{\beta}c^{\beta}_i],
$](img51.png)
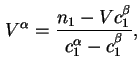
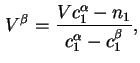
For liquid/vapour interfaces the following approximate equation may be used in the domain of low vapour pressures:
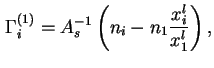
The reduced adsorption (
![]() ) of component
) of component ![]() is defined by the equation
is defined by the equation
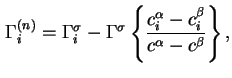
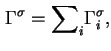
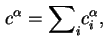
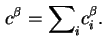
The reduced adsorption is also invariant to the location of the Gibbs surface.
Alternatively, the reduced adsorption may be regarded as the Gibbs surface concentration of ![]() when the Gibbs surface is chosen so that
when the Gibbs surface is chosen so that
![]() is zero, i.e. the Gibbs surface is chosen so that the reference system has not only the same volume, but also contains the same total amount of substance (
is zero, i.e. the Gibbs surface is chosen so that the reference system has not only the same volume, but also contains the same total amount of substance (![]() ) as the real system.
) as the real system.
Hence
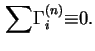
In terms of experimental quantities
![$\displaystyle {\Gamma}_i^{(n)} = A_s^{-1}[n_i - V^{\alpha}c^{\alpha}_i- V^{\beta}c^{\beta}_i],
$](img68.png)
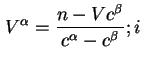
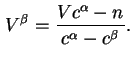
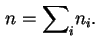
![]() and
and ![]() thus defined correspond to
thus defined correspond to
![]() .
.
For liquid/vapour interfaces the following approximate equation may be used in the domain of low vapour pressures:
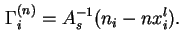
Because both
![]() and
and
![]() are invariant to the position of the Gibbs surface, it is possible to dispense with the concept of the Gibbs surface and to formulate the above definitions without explicit reference to a dividing surface.
are invariant to the position of the Gibbs surface, it is possible to dispense with the concept of the Gibbs surface and to formulate the above definitions without explicit reference to a dividing surface.
It may happen that component ![]() is virtually insoluble in both of the adjoining phases, i.e.
is virtually insoluble in both of the adjoining phases, i.e.
![]() , but is present as a monolayer between them. Such a layer can be produced by spreading and is called a spread monolayer11. The relative and reduced adsorption become indistinguishable for such a component as does the difference between surface excess amount (
, but is present as a monolayer between them. Such a layer can be produced by spreading and is called a spread monolayer11. The relative and reduced adsorption become indistinguishable for such a component as does the difference between surface excess amount (
![]() ) and amount of adsorbed substance
) and amount of adsorbed substance ![]() , (see §1.1.11). In this case the
surface concentration (= surface excess concentration) is
defined by
, (see §1.1.11). In this case the
surface concentration (= surface excess concentration) is
defined by
The symbol for the (average) area per molecule (in the surface)12 is ![]() or
or ![]() .
.