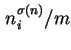 (
(
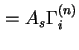 ), or
), or
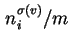 (
(
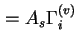 ) are used when the surface area of the solid is not known with certainty.
) are used when the surface area of the solid is not known with certainty.
For solid/liquid systems, two different definitions of the surface excess amount,
![]() and
and
![]() , are frequently used. When the surface area of the solid is known, then these may be expressed as the surface excess concentrations
, are frequently used. When the surface area of the solid is known, then these may be expressed as the surface excess concentrations
![]() , (reduced adsorption), or
, (reduced adsorption), or
![]() (for which no specific name has been proposed), each relating to a particular procedure for calculating adsorption from solution. The corresponding specific quantities,
(for which no specific name has been proposed), each relating to a particular procedure for calculating adsorption from solution. The corresponding specific quantities,
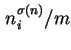 (
(
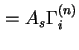 ), or
), or
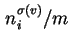 (
(
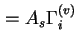 ) are used when the surface area of the solid is not known with certainty.
) are used when the surface area of the solid is not known with certainty.
![]() is the excess, per unit area of solid/liquid interface, of the amount of component
is the excess, per unit area of solid/liquid interface, of the amount of component ![]() in the system, over the amount of
in the system, over the amount of ![]() in a reference system containing the same total amount
in a reference system containing the same total amount ![]() , of liquid and in which a constant mole traction
, of liquid and in which a constant mole traction ![]() equal to that in the bulk liquid in the real system, is maintained throughout the liquid phase:
equal to that in the bulk liquid in the real system, is maintained throughout the liquid phase:
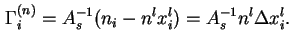
It follows from the above definition that the total reduced surface excess of components in the liquid phase is zero:
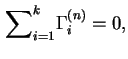
The above definition of
![]() is essentially algebraic (cf. end of first paragraph in §1.1.8), and is independent of the choice of a Gibbs dividing surface. It may be noted, however, that the Gibbs surface corresponding to this definition does not in general, coincide with the surface of the solid and, consequently
is essentially algebraic (cf. end of first paragraph in §1.1.8), and is independent of the choice of a Gibbs dividing surface. It may be noted, however, that the Gibbs surface corresponding to this definition does not in general, coincide with the surface of the solid and, consequently
![]() according to this interpretation.
according to this interpretation.
Similarly, as in the case of the fluid/fluid interface, it may sometimes be expedient to define and calculate from experimental data the relative adsorption,
![]() , as the excess, per unit area of solid/liquid interface, of the amount of component
, as the excess, per unit area of solid/liquid interface, of the amount of component ![]() in the actual system, over the amount of
in the actual system, over the amount of ![]() in a reference system containing the same amount of component 1 as the real system and in which a constant composition, equal to that of the bulk liquid in the real system, is maintained throughout the liquid phase:
in a reference system containing the same amount of component 1 as the real system and in which a constant composition, equal to that of the bulk liquid in the real system, is maintained throughout the liquid phase:
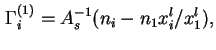
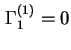 , by definition. Note that for a binary system
, by definition. Note that for a binary system
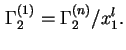
Analogous and completely equivalent definitions of reduced and relative adsorption may be formulated and used in terms of masses and mass fractions, respectively.
![]() is the excess, per unit area of solid/liquid interface, of the amount of component
is the excess, per unit area of solid/liquid interface, of the amount of component ![]() in the system over the amount of component
in the system over the amount of component ![]() in the reference system containing the same volume
in the reference system containing the same volume ![]() , of liquid and in which a constant concentration, equal to that in the bulk liquid in the real system, is maintained throughout the liquid phase.
, of liquid and in which a constant concentration, equal to that in the bulk liquid in the real system, is maintained throughout the liquid phase.
![]() is defined as the difference between the total volume of the system and that of the solid, assuming that the latter is not changed by the adsorption process.
is defined as the difference between the total volume of the system and that of the solid, assuming that the latter is not changed by the adsorption process.
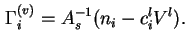
If it is assumed that the volume of the liquid including adsorbed material is unchanged by contact with the solid, then
![]() where
where ![]() is the initial concentration of
is the initial concentration of ![]() in the liquid before contact with the solid, and
in the liquid before contact with the solid, and
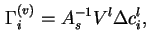
If it is assumed that ![]() , the partial molar volume of
, the partial molar volume of
![]() in the liquid, is independent of concentration and adsorption, the Gibbs surface concentrations of the various components defined in this way are related by the equation
in the liquid, is independent of concentration and adsorption, the Gibbs surface concentrations of the various components defined in this way are related by the equation
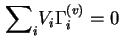
Because the calculation of
![]() from experimental measurements is based on the assumption of constant total volume, it is advisable to confine its use, and that of the corresponding specific quantity
from experimental measurements is based on the assumption of constant total volume, it is advisable to confine its use, and that of the corresponding specific quantity
![]() , where
, where ![]() is the mass of adsorbent, to practically ideal solutions, and in particular to ideal dilute solutions.
is the mass of adsorbent, to practically ideal solutions, and in particular to ideal dilute solutions.
The surface excess isotherm is the function relating, at constant temperature and pressure,
![]() ,
,
![]() or
or
![]() , or the respective specific quantities
, or the respective specific quantities
![]() ,
,
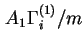 or
or
![]() to the mole fraction (or concentration) of component i in the equilibrium liquid phase. With solutions of more than two components such isotherms are unequivocal functions only when the ratios of the mole fractions (or concentrations) of all other components except
to the mole fraction (or concentration) of component i in the equilibrium liquid phase. With solutions of more than two components such isotherms are unequivocal functions only when the ratios of the mole fractions (or concentrations) of all other components except ![]() are kept constant.
are kept constant.
The term composite isotherm has been used as a synonym for surface excess isotherm, but is not recommended.
The term individual isotherm or partial isotherm is the function relating, at constant temperature and pressure, the amount of a particular component in the interfacial layer per unit area (or per unit mass of adsorbent) with its mole fraction (or concentration) in the liquid phase. This function
![]() (or
(or
![]() can be evaluated only when the location and the thickness of the interfacial layer has been defined. In this case, the surface excess can be expressed as
can be evaluated only when the location and the thickness of the interfacial layer has been defined. In this case, the surface excess can be expressed as
![]() (or
(or
![]() ), where
), where
![]() is the total amount of substance in the interfacial layer (and
is the total amount of substance in the interfacial layer (and ![]() is its volume). The amount
is its volume). The amount ![]() thus becomes identical with the experimentally accessible surface excess when the equilibrium concentration of
thus becomes identical with the experimentally accessible surface excess when the equilibrium concentration of ![]() in the liquid is negligibly small.
in the liquid is negligibly small.
In connection with strongly adsorbed solutes of limited solubility, the value of ![]() reached in a saturated solution is called the adsorption capacity of the adsorbent for solute
reached in a saturated solution is called the adsorption capacity of the adsorbent for solute ![]() ; its value depends also, in general, on the nature and, in the case of more than two components, on the relative composition of the bulk liquid.
; its value depends also, in general, on the nature and, in the case of more than two components, on the relative composition of the bulk liquid.