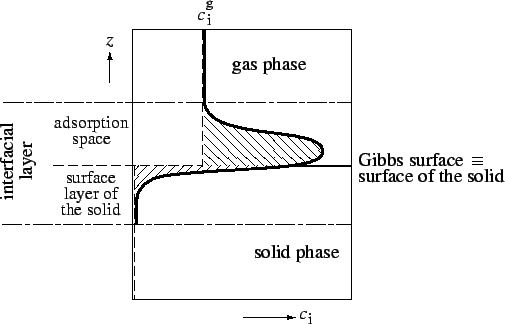
Surface excess amount of adsorbed substance (Gibbs
adsorption), (
![]() ) is the excess of the amount of component
) is the excess of the amount of component ![]() actually present in the interfacial layer over that which would be present at the same equilibrium gas pressure in the reference system, in which the gas phase concentration is constant up to the Gibbs surface, and the reference concentration of component
actually present in the interfacial layer over that which would be present at the same equilibrium gas pressure in the reference system, in which the gas phase concentration is constant up to the Gibbs surface, and the reference concentration of component ![]() is zero beyond the Gibbs surface in the surface layer of the solid (see Figure 2).
is zero beyond the Gibbs surface in the surface layer of the solid (see Figure 2).
The general expression for
![]() (§1.1.8) becomes in this instance:
(§1.1.8) becomes in this instance:
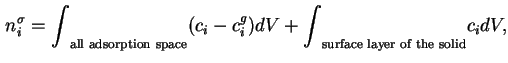
For a multicomponent gas mixture the total surface excess amount of adsorbed substance is
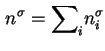
If the area ![]() of the solid surface is known, then the
surface excess concentration (or Gibbs surface
concentration) of component
of the solid surface is known, then the
surface excess concentration (or Gibbs surface
concentration) of component ![]() , denoted by
, denoted by
![]() , is
, is
Similar definitions can be given for the surface excess number of molecules of component ![]() ,
,
![]() , and of the surface excess mass of
, and of the surface excess mass of ![]() ,
,
![]() , and of the surface excess volume of gas of
, and of the surface excess volume of gas of ![]() (
(
![]() ) preferably expressed as the volume of gas calculated for 273.15 and 101.325 (0 and 1 atm): the equation of state used in the calculation should be stated.
) preferably expressed as the volume of gas calculated for 273.15 and 101.325 (0 and 1 atm): the equation of state used in the calculation should be stated.
The operational definition of
![]() is
is
|
The amount of adsorbed substance is defined as
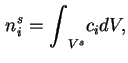
The following definitions refer to the adsorption of a single adsorptive.
Adsorption isotherm, in the case of a single adsorptive,
is the function relating the amount, mass or volume, or corresponding excess of substance adsorbed by a given amount of solid to the equilibrium pressure (![]() ) at constant temperature (
) at constant temperature (![]() )14
)14
Adsorption isobar is the function relating the amount, mass, or volume, or corresponding excess of substance adsorbed by a given amount of solid to the temperature at constant pressure.
Adsorption isostere is the function relating the equilibrium pressure to the temperature at a constant value of the amount, or excess amount, of substance adsorbed by a given amount of solid.
When the specific surface area (![]() ) is measured by adsorption methods15 then it is given by the product of the
specific monolayer capacity,
) is measured by adsorption methods15 then it is given by the product of the
specific monolayer capacity, ![]() , the Avogadro
constant, and the area occupied by a molecule adsorbed in a complete monolayer (
, the Avogadro
constant, and the area occupied by a molecule adsorbed in a complete monolayer (![]() ):
):
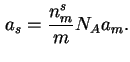
In the case of microporous solids the interpretation of adsorption measurements in terms of surface area may lose its significance when the size of the adsorbed molecules is comparable with the dimensions of the pores. Nevertheless it may be convenient to define a monolayer equivalent area, in which ![]() is replaced in the above equation by the amount needed to fill the micropores (§1.1.7).
is replaced in the above equation by the amount needed to fill the micropores (§1.1.7).