ST11
Separation of heterogeneous solid - solid mixtures
| Aim:
To become acquainted with two separation techniques for heterogeneous
solid - solid mixtures. |
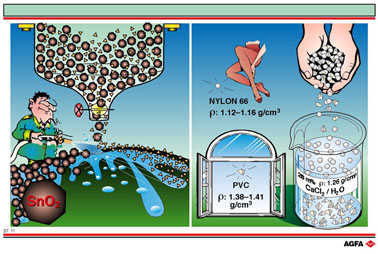
The left-hand
side of illustration ST 11 shows an example of sluicing. In the sluicing
process a regulated water spray is used to separate off lighter particles
(the gangue) from heavier ore particles. In this example as much gangue
as possible is being removed from cassiterite ore (tin oxide ore).
The right hand side of the illustration shows a mixture of plastics being
separated on the basis of the difference in density between the various
types. The example shows the separation of a mixture of polyvinyl chloride
(PVC) and Nylon 66. A 28% w/w solution of CaCl2 in water has
a density of 1.26 g/cm3. When a mixture of PVC/Nylon is added
to it the Nylon floats and the PVC sinks.This separation occurs because
the density of Nylon (1.12 - 1.16 g/cm3) is lower than that
of the CaCl2 solution, and the density of PVC
(1.38 - 1.41 g/cm3) is higher than that of the CaCl2
solution. This method is of practical use during the separation ofvarious
plastics for recycling.
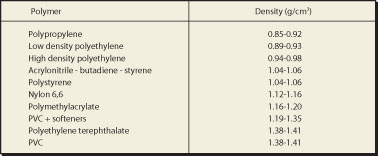
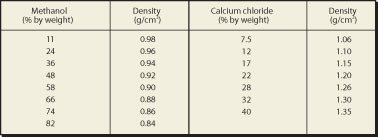
In the table below a brief overview is given of certain simply made solutions that can be used for separation of various mixtures of plastics. The table gives the density of aqueous solutions of methanol and CaCl2 at 20°C.