ST12
Separation of heterogeneous solid - solid mixtures
| Aim:
To show the flotation process, that is frequently used to enrich
sulphide ores. |
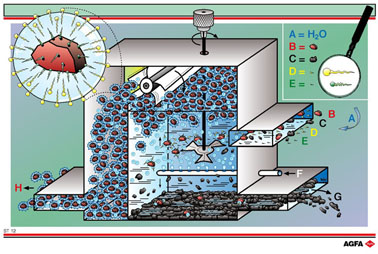
Illustration
ST12 shows a schematic diagram of a flotation unit. ‘Flotation’
involves the separation of a heterogeneous solid - solid mixture, e.g.
a mixture of sulphide ore and gangue. The method relies on the addition
of surfacetension- reducing substances (D) (surface-active agents, e.g.
soaps) to an aqueous suspension (A) of a mixture of oreand gangue (B +
C) to which a flotation agent (E), e.g. oil, has already been added. The
flotation agent influences the selectivity of the wetting between the
ore and the gangue.
The ore selectively adsorbs the flotation agent, so that the surfactant
can dampen it more easily.
Surface
active agents are composed of an apolar tail and a polar head. The ore
particles are covered by the flotation agent and this adsorbs the apolar
tails of the surfactant, resulting in the ore being wetted. At the same
time the polar heads point outward and are attracted by the water molecules.
When air is blown in (F) the presence of the surfactants gives rise to
foam formation. In the aqueous suspension rising air bubbles encircle
the wetted ore-particles.
This gives rise to ore particles that are covered with air bubbles. On
the surfaces of these ore-air bubbles the polar heads of the surfactant
again point towards the aqueous phase. The apolar tails point towards
the ore (see top left of the illustration). The fact that the ore particles
are attached to air bubbles causes them to rise to the top, into the foam
layer. This foam is skimmed off the water surface by a bladed wheel (H).
The less-wetted gangue sinks to the bottom of the tank (G).