CG17 Opiates: painkillers derived from morphine
| Aim:
To illustrate the relationship between the three dimensional structure
of a medicine and its working, using morphine as an example. |
The term
opiates covers an important class of painkillers, chiefly used in the
treatment of deep chronic pain, that influence the central nervous system.
Opiates are probably the oldest known medicine: they were already in use
in China 2000 years ago.
The source of opiates is to be found in opium alkaloids.
These are natural products extracted from opium, the sticky discharge
of the poppy (papaver somniferum) that contains more than 25 alkaloids.
The term alkaloid points to a range of biologically active natural products,
all of which contain a nitrogen atom. The alkaloid that works as a painkiller
in opium is morphine called after the Roman god of sleep, Morpheus. When
morphine was synthesized in 1952 it became possible to use it in a pure
form; a number of side effects were then immediately obvious, the most
important being constipation, a feeling of euphoria, tolerance, dependence
and effects on breathing.
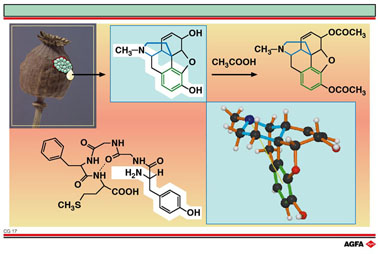
Morphine
consists of five rings and has a pronounced
T-form.
It is basic (tertiary amino group) and also contains a phenol group, an
alcohol group, an ether bridge and a double bond.
The aromatic ring and the basic character of the nitrogen (shown with
a white background on the slide) together with the various chiral centres
are essential for morphine's activity. For an optimal interaction with
the receptor the aromatic ring has to be correctly orientated with respect
to the nitrogen atom.
The three-dimensional structure of morphine derivatives, determined by
X-ray diffraction, exhibits, among others, the following properties:
- the angle between the nitrogen containing ring (blue)
and the aromatic ring (green) is about 80°.
- the distance between the nitrogen atom and the
centre of the aromatic ring (shown by the yellow
line) is about 0.46 nm.
- the
perpendicular distance between the nitrogen atom and the
plane containing the aromatic ring is about
0.07 nm.
When looking for better painkillers, morphine was the model for many synthetic
variants. Both hydroxyl groups in morphine can undergo esterification
whereby morphine diacetate, better known as heroin, is obtained (upper
right). Heroin is twice as effective as morphine as it can reach the brain
more easily. The blood - brain barrier that prevents the invasion of the
brain by various types of substances is most effective for polar compounds.
In heroin the acetyl groups protect the two polar hydroxyl groups of morphine,
and so heroin can more easily penetrate the blood-brain barrier. Heroin
is considered to be a pro-drug of morphine; the acetyl groups are split
off again in the brain by esterases.
Given that opiates work directly on receptors in the central nervous system
and in this manner raise the pain barrier, natural painkillers must also
be present in our bodies.
Encephalines fulfil this role. A typical representative, Metencephaline
(bottom left) is a pentapeptide with the amino acid sequence Tyr-Gly-Gly-Phe-Met.
This chain folds, due to intramolecular hydrogen bonding, in such a way
that a further interaction arises between the aromatic ring of phenyalanine
and the receptor.