CG18 Sulphamides: a school example of organic synthesis
| Aim:
give an example of the various steps involved in industrial organic
synthesis of a medical drug. |
Sulphanilamide
was first prepared in 1908 but its therapeutic value was only recognised
in 1932.
Numerous derivatives have been synthesised since then. It can be seen
as a milestone in the history of chemotherapy because the synthesis of
"sulpha" medicines signified the first rational study for the
synthesis of organic compounds.
The various steps in the synthesis can be described as follows:
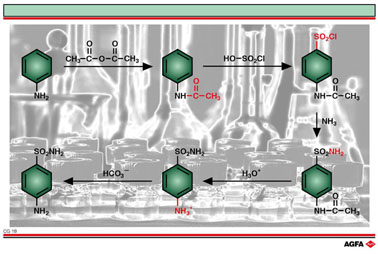
- The reaction
starts from aniline, which must be
sulphonated. To protect the amino-group, without
losing the para-directing effect, the amino-group is
first acetylated to acetanilide using acetic anhydride:
i.e. nucleophilic substitution of the amino group by the
anhydride.
- By electrophilic substitution of an acid chloride
(chlorosulphonic acid) into the aromatic ring, an
aromatic sulphonyl chloride is formed. The N-acetyl-
group maintains its ortho-para-directing action.
- Organic sulphonyl-chlorides react with ammonia to
form sulphonamides: i.e. nucleophilic substitution of
ammonia in the acid chloride.
- All that remains is to unblock the amino group. This
can be achieved by acid hydrolysis of the protecting
amide group (nucleophilic substitution).
The sulphamide group remains stable.
- The amino group, that was protonated in the acidic
solution, is recovered by an acid-base reaction using a basic
solution of bicarbonate ions.