CG16 Sulphamides: the success of chemical inhibition
| Aim:
To illustrate medicines that work by reversible inhibition of enzymes. |
The
key to the discovery of the working of sulphanilamide was found in 1940
when it was discovered that the inhibition of bacterial growth by sulphanilamide
could be reversed by the addition of large quantities of p-amino benzoic
acid (PABA). When it was further realised that PABA was an essential growth
factor for certain bacteria, the effect could be explained as a competition
between sulphanilamide and PABA.
Sulphanilamide and p- amino benzoic acid have many structural similarities.
This results in them being able to interfere with each other when bonding
with enzymes that are responsible for the synthesis of the vitamin folic
acid in bacteria.
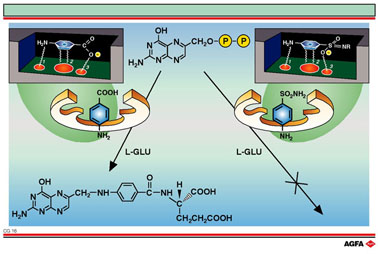
The folic acid (below) is built up, with the help of enzymes, by linking
three molecular fragments:2 - amino – 4-hydroxy - 6 - methylpteridine
(upper centre), L-glutaminic acid and p-amino benzoic acid. If sulphanilic
acid instead of PABA binds onto the bonding-point on the enzyme, there
is no resultant folic acid synthesis.
Humans require folic acid too, but they obtain it from food.