R20 Electrolytic extraction and purification of metals
Aim: To describe the installations and processes used to extract one metal (Al) from its ore and to purify another (Cu). |
Electrolysis is frequently
used to extract a metal from its ore, or to purify an impure metal.
1. Electrolytic extraction of metals.
The commonest metal ores contain the metals as oxides or carbonates. The metal can in
theory be obtained by the electrolytic reduction of the ores. However, in practice
chemical reduction using carbon or sulphur is more often used because it is cheaper, but
this produces an impure metal and can cause environmental problems. When product purity is
important, electrolysis is the preferred method. Al, Co, Cr, Mn, Ta, and Mg are all
extracted using electrolytic processes.
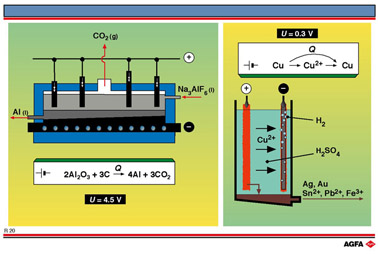
Illustration R20 schematically shows an electrolytic process for the extraction of Al from
its ore (bauxite, an oxide of aluminium containing silicon and other impurities). This
process is used to produce 25 million tons of aluminium per year. After chlorine, this is
the most important product of the electrochemical industry.
Aluminium is in great demand for the automobile, shipbuilding, aircraft, electrotechnical
and building industries and, since there are ample reserves of bauxite, the future for the
aluminium industry seems bright.
The electrolytic extraction process of aluminium from bauxite was originally developed by
Hall (USA) and Héroult (France) in 1886 and improved in 1887 by Bayer (Germany).
The first
step in this process is the dissolution of bauxite in sodium hydroxide under pressure as
sodium aluminate. This is a self-sustaining reaction, which does not involve electron
transfer. The SiO2 and Fe2O3 impurities are precipitated
and removed at this stage.
![]()
The next step is the precipitation of aluminium oxide from the sodium aluminate solution.
This is achieved by dilution with water, seeding with solid aluminium(III) hydroxide or
treating with carbon dioxide.
The precipitated aluminium hydroxide is then separated off and heated to obtain aluminium
oxide, which is then dissolved in molten cryolite (Na3AlF6).
![]()
where Q indicates the consumption of electricity.
Aluminium is discharged at the cathode and oxygen is evolved at the anode,
which oxidises the graphite anode to carbon dioxide.
The appropriate half-reaction equations are:
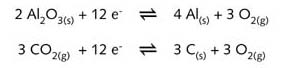
The second
equation contains a multiple of 3 in order to balance the electrons in
the two half-equations.
This process is operated under the following conditions:
- Voltage = 4.5 V (cf. theoretical value of 2.2 V).
- Current = 150 kA
- Cell size = 3 x 8 x 0.7m, containing 8 graphite blocks (200 single cells, each 15 m3, in series).
- 15 MWh of electricity are required to produce 1 ton of aluminium, which is 5 times more electricity than is required to produce 1 ton of chlorine.
2. Electrolytic refining of metals.
Impure metals can be purified by electrolysis. In an electrolytic cell
the anode is made from the crude metal needing to be purified, the cathode
from the purified metal. The electrode potential is selected to ensure
the very selective reduction of the metal at the cathode.
During the transfer of the metal from the impure metal of the positively
charged anode to the negatively charged cathode the impurities remain
behind in the electrolyte solution. The electrolyte is chosen according
to which element is to be purified. For Cu, Ag, Au and Pt, aqueous solutions
are used, whereas for Na, Mg, Ca and Al molten salts are employed.
Illustration R20 illustrates the electrolytic refining of copper. Impure
copper is the anode, pure copper the cathode and an aqueous mixture of
sulphuric acid and copper sulphate is the electrolyte. The electrode potential
is carefully selected so that only copper is reduced at the cathode.
This is the most important method of copper purification, producing 100,000
tons of purified Cu per year. It is based on the following considerations:
- Ag, Au and Pt, all precious metals, have a lower reduction potential than Cu. They are not oxidised to ions at the anode. As the copper ions are formed, the anode crumbles away allowing the precious metal impurities to fall as sludge to the bottom of the cell.
- Sn, Bi and Sb have a larger reduction potential than Cu. They are therefore oxidised at the anode but their ions react with the electrolyte to form insoluble oxides and hydroxides. These are also deposited in the sludge.
- Pb is also oxidised, but forms insoluble PbSO4, which again sinks into the sludge.
- Fe, Ni, Co and Sn are oxidised at the anode, but remain as ions in the electrolyte. This is a consequence of the careful choice of electrode potential, which ensures that only the copper ions are reduced at the cathode, the other metal ions needing a higher electrode potential to be reduced.
Electrolytic methods are also used to recover the precious metals from the anodic sludge.