R18 - R19 Producing chlorine in the chlorine-alkali industry: the mercury cell - diaphragm-, and membrane-cells
Aim: To describe schematically processes used world-wide for the electrolytic production of NaOH and Cl2, two of the most important basic industrial chemicals. |
The chlorine-alkali
industry is an important branch of the chemical industry, producing chlorine and sodium
hydroxide by the electrolysis of common salt. The main raw material is brine, a saturated
aqueous solution of sodium chloride (NaCl) obtained from natural salt deposits.
50
Million tons of Cl2 are produced annually. Whilst some of this is marketed as
chlorine gas, e.g. to disinfect drinking water and swimming pools, most of it is used in
the synthesis of (poly)vinylchloride and other chlorohydrocarbons, such as
chlorohydrocarbon solvents, cooling liquids, insecticides, pesticides, fungicides etc.
Electrolytic production of chlorine also generates two useful by-products: sodium
hydroxide, and hydrogen.
The half-reaction equations give an interesting insight into the process:
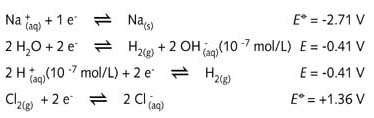
The
concentrations of the H+ and OH- are those for neutral water and the
E value is therefore -0.41 V.
In theory
chlorine could be produced by reacting Cl- ions with Na+, H+,
or H2O molecules; all three are above and to the left of Cl2 in the
list of standard reduction potentials . None of these reactions would be self-sustaining.
For them to proceed, a constant source of electrical energy would be required: i.e. an
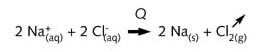
electrolytic process would be needed. An electrolysis process, in which Cl-
ions reduce Na+ ions, would, in theory, give rise not only to Cl2,
but also Na(s). The sodium metal would, however, immediately react with any water and/or H+
ions present in the cell to produce hydrogen gas and OH- ions (i.e. NaOH
solution).
Such a
process would be very useful, since three important chemicals would be produced during a
single process. However, there is a problem since the chlorine, produced in the presence
of a basic solution of sodium hydroxide, would combine with it to form ClO-
ions and Cl- ions. This results in the production of sodium chlorate(I), NaClO,
a component of household bleach. To overcome this problem the chlorine and sodium
hydroxide must be removed from the cell before they can react.
Three industrial electrolytic processes are currently used for the production of chlorine,
all of which have overcome the above-mentioned problem.
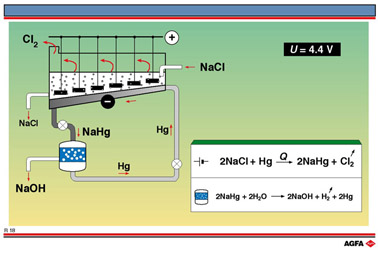
1. The mercury cell.
This cell, schematically shown in illustration R18, is used in the electrolytic production
of both sodium hydroxide and chlorine from a saturated solution of brine and operates at
4.4 V. Electrolysis of brine would normally generate hydrogen at the cathode, but if
mercury is used as the cathode material this does not occur. At a negatively charged
mercury cathode hydrogen has a high overvoltage, meaning that a higher negative potential
is required for the discharge of hydrogen ions. At the same time the discharge potential
of Na+ ions is lowered, since the sodium atoms combine with mercury to form an amalgam.
This also protects the sodium from contact and therefore reaction with water so the
problem of the alkaline medium is avoided.
Chlorine is produced at the positively charged anode, which traditionally consisted of a
series of suspended graphite rods but are now being replaced by more expensive, but more
durable ,Ti or Pt-steel alloys. The chorine which is formed collects at the top of the
cell.
The sodium amalgam is run off from the bottom of the cell into a separate chamber
containing graphite balls.
The graphite catalyses the self-sustaining dissociation of sodium amalgam. The sodium
released reacts with water to form sodium hydroxide and hydrogen, the mercury being
recovered and returned to the electrolysis cell.
![]()
The brine consumed in the electrolyte cell is continually replenished. The overall reaction is:
Industrial installations
consist of some 200 mercury cells in series, each one measuring 15 m x 2 m x 0.3 m. The
process, which takes place at a very high voltage, uses an enormous quantity of
electricity: 3 MWh per ton Cl2.
This method only produces a fraction of the chlorine and sodium hydroxide used by industry
as it has certain disadvantages: mercury is expensive and toxic, and whilst it is
recirculated, some always escapes with the spent brine with which it reacts to form
mercury(II) chloride.
In the past this effluent was discharged into lakes and rivers, leading to the
accumulation of high levels of mercury in fish, which absorbed the mercury compound but
could not re-excrete it. Nowadays the spent brine is treated before discharge, the mercury
being precipitated as mercury(II) sulphide.
In recent years a large share of chlorine and sodium hydroxide production has been
produced in two other types of cell, which do not use mercury: the membrane cell and the
diaphragm cell. In the UK 1 diaphragm cell is in use for every 20 mercury cells and in the
USA 2 diaphragm cells are in use for every mercury cell.
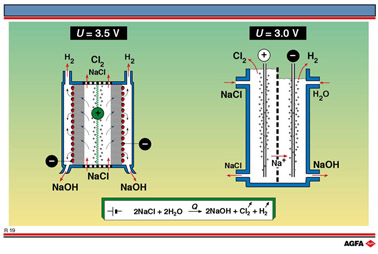
2. The diaphragm
cell.
This is illustrated on the left of illustration R19. On the negatively charged cathode
surface NaOH and H2 are formed directly and Cl2 is formed at the
anode. To separate the chlorine from the sodium hydroxide, the two half-cells were
traditionally separated by a porous asbestos diaphragm, which needed to be replaced every
two months. This was environmentally detrimental owing to the need of disposing of large
quantities of asbestos. Such frequent replacement is fortunately no longer necessary, the
asbestos having now been replaced in part by polymers resulting in diaphragms with a much
longer life.
The anode is made of a titanium-steel alloy, and the cathode of steel. Calcium- and
magnesium-ion impurities must be removed from the brine before it is electrolysed,
otherwise they will precipitate out as insoluble hydroxides and block the pores of the
diaphragm. To ensure seepage of brine through the diaphragm from the anode to the cathode,
the level is kept higher in the anode compartment. The diaphragm cell is now
technologically the most advanced of all three cells and has a high electrochemical
performance.
3. The membrane cell.
A membrane cell is illustrated on the right of illustration R19. This is very similar to
the diaphragm cell, and the same reactions occur. The main difference is that the two
electrodes are here separated by an ion-selective polymer membrane which only allows
cations to pass through, instead of an asbestos diaphragm. Brine is pumped in at the top
of the anode compartment, and water is introduced at the top of the cathode compartment.
At the
negatively charged cathode, hydrogen ions in the water are reduced to hydrogen gas. At the
positively charged anode Cl- ions from the brine are oxidised to Cl2
gas. The Na+ ions flow through the membrane to the cathode compartment, thereby
carrying the current and form NaOH with the leftover OH- ions. The chloride
ions cannot pass through, so the chlorine does not come into contact with the sodium
hydroxide which is formed in the cathode compartment. The sodium hydroxide is removed from
the bottom of the cell.
The overall reaction for the diaphragm and membrane cells is:
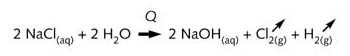
The
advantage that the two membrane cells have (relative to the mercury cell) is that the Cl2-gas
and NaOH do not come into contact with each other. They also use less electricity and are
therefor cheaper to operate.
Reference :
Buchner, Schliebs, Winter and Büchel, Industrial Inorganic Chemistry, VCH, Weinheim,
Germany.