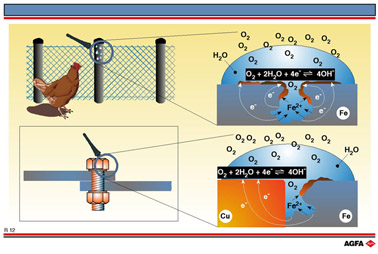
R12 Atmospheric corrosion
Aim: To show how atmospheric corrosion occurs and which redox half-reactions are responsible for it |
Corrosion has posed a
problem for centuries and is evident in the home, garden, transport vehicles (from
bicycles to cars), shipping, industry and underground piping. The consequences of
corrosion are all too familiar, parts have to be replaced, customers become dissatisfied
and there are other adverse financial consequences. 1/8th of the annual UK production of
steel is needed to replace iron lost through rusting.
Combating of corrosion often requires expensive surface treatment e.g. painting,
galvanisation, tinning etc., which often is associated with products which themselves
cause serious contamination of land, water and air.
Rusting of iron.
Corrosion is a widespread problem which can be explained in terms of redox reactions as
represented on illustration R12. The top right-hand side shows an iron surface which is in
contact with water containing a little dissolved oxygen.
All the necessary reagents are present to form a corrosion cell. (This is a simple
electrochemical cell in which corrosion occurs.) The etched out corrosion cell depicted
has an anodic area, a cathodic area, a suitable transport medium for electrons (the metal
itself) and an aqueous solution through which ions can move. The following electrochemical
half-reactions are important when considering how the anodic and cathodic areas are
formed:
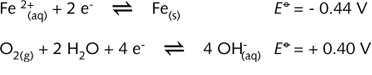
From
the above ![]() -values
it can be deduced that Fe, the metal surface, is the anode. The top of
the water droplet is in contact with the oxygen in the air and so the
concentration of oxygen is greater here than in the water droplet itself.
This region of higher concentration is the main cathodic zone. The Nernst
equation (R8) shows that the oxidising potential increases with an increase
in oxygen partial pressure.
-values
it can be deduced that Fe, the metal surface, is the anode. The top of
the water droplet is in contact with the oxygen in the air and so the
concentration of oxygen is greater here than in the water droplet itself.
This region of higher concentration is the main cathodic zone. The Nernst
equation (R8) shows that the oxidising potential increases with an increase
in oxygen partial pressure.
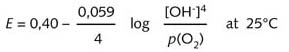
![]()
The iron(II) ions which are formed in the water come into contact with the rapidly diffusing hydroxide ions and react to form insoluble iron(II) hydroxide. This is in turn oxidised by the air to iron(III) oxide.
![]()
The
cathode or cathodic zone is more than just the iron metal. Iron together with the oxides
FeO, Fe2O3, and hydroxides Fe(OH)2 and Fe(OH)3
forms the complex cathodic surface which is called rust.
Constant diffusion takes place between the anodic and cathodic areas and, because the
cathode receives its oxygen supply from the air, this type of corrosion is called
atmospheric corrosion.
A few characteristics of the rusting process are:
• The presence of salt in the water leading to a greater
degree
of corrosion, because it is ionic and when
dissolved
in water its ions encourage the transport of
ions
already within the system.
• Corrosion
only continuing if the rust which is formed
conducts
electrons. For iron this is the case but if the iron
is incorporated into a steel alloy e.g. stainless
steel
( 18% Cr, and 8% Ni ) then an insulating layer
of
CrO2
forms on the surface of the iron and if a
rusting
process
starts it stops almost immediately due to
lack of
electron transport.
• Presence
of an acid results in H+ ions from the acid
reacting
with the hydroxide ions produced during
corrosion
to form water. This will result in the
formation
of more hydroxide ions and more corrosion.
Corrosion at the junction of copper and iron
When two metals, one of which is iron, are joined together it is important that corrosion
of the iron is prevented. At the bottom left-hand side of R12 a copper bolt has been used
to join two iron plates. Copper metal has a much lower reducing power than iron. The redox
couples to be considered are:

The values of ![]() are very similar. In non-standard situations the order can even be reversed
and the oxidizing and reducing power lost. Copper corrodes very little
but is an excellent conductor of electrons. It therefore provides an excellent
cathodic surface in the presence of iron ,thus increasing the corrosion
of the iron. The copper bolt still exists after the two iron plates have
corroded away!
are very similar. In non-standard situations the order can even be reversed
and the oxidizing and reducing power lost. Copper corrodes very little
but is an excellent conductor of electrons. It therefore provides an excellent
cathodic surface in the presence of iron ,thus increasing the corrosion
of the iron. The copper bolt still exists after the two iron plates have
corroded away!