R13 Combating corrosion: surface treatment
Aim: To illustrate the advantages and disadvantages of surface treatment in combating corrosion |
There are
various ways in which corrosion can be combated. These involve changing:
1.
the anode surface or the anodic half-reaction.
2.
the cathode surface
3.
the electrolyte
4.
the nature of the oxidising agent
5.
the ability to transport electrons
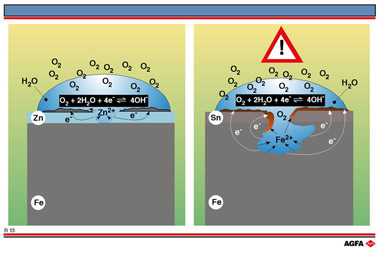
The anodic surface can be changed by protecting the metal with a thin
layer of paint (lacquering), zinc (galvanising) or tin (tinning).
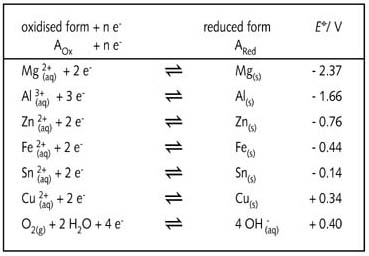
When considering illustration R13, it is necessary to bear in mind the
table of standard reduction potentials for the half-reactions involved
in the process.
Standard reduction potentials for the half-reactions considered in
illustrations R12 and R13.
the more negative
Furthermore, even when microscopic defects are present in the zinc layer an electrochemical cell is set up with the more strongly reducing Zn2+/Zn redox couple as an anode and the Fe2+/Fe redox couple as the cathode resulting in the zinc anode being preferentially oxidised, so protecting the iron underneath and preventing it from rusting.
Although the layer of zinc is very thin the protection is good for a long time.
In the food industry iron is protected by a very thin layer of tin in tin cans. The