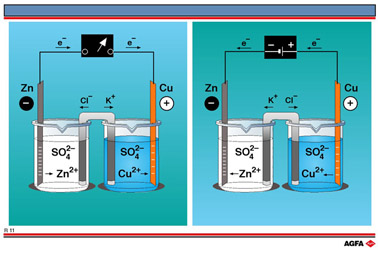
R11 Electrolytic cell versus electrochemical cell
Aim: To illustrate the relationship between a electrochemical cell and an electrolytic cell or between a self-sustaining redox reaction and a sustained redox reaction. |
Electron
transfer reactions, like most chemical reactions, can in principle proceed
in either direction according to the equation or even in both directions
at once. In an electrochemical cell the electrode reactions are
selfsustaining proceeding until one reactant is consumed or until the
reduction potentials of the two half-cells become equal. In an electrolytic
cell, on the other hand, electrical energy is supplied which sustains
a redox reaction that would otherwise not take place : a so-called sustained
redox reaction.
Cells, whether they be electrochemical or electrolytic, are by convention always shown
with the negative electrode on the left-hand side i.e. in electrochemical cells electron
flow in self-sustaining redox reactions is always depicted from left to right, whereas the
flow of electrons supplied to a redox reaction is always depicted from right to left.
In illustration R11 two cells are shown
with Zn2+/Zn and Cu2+/Cu redox couples in their half-cells. An
electrochemical cell configuration is depicted on the left-hand side, a Daniell cell in
which electrons flow from the half-cell with the Zn2+/Zn redox couple to that
with the Cu2+/Cu redox couple in a self-sustaining redox reaction
which proceeds until either the zinc metal or the copper(II) ions have been consumed.
An
electrolytic cell is depicted on the right-hand side of illustration R11 in which
electrons, supplied by a battery, flows from the half-cell with the Cu2+/Cu
redox couple to that with the Zn2+/Zn redox couple until either the copper
metal or the zinc(II) ions have been consumed in a sustained redox reaction.
The redox reactions taking place in these cell are summarised below:
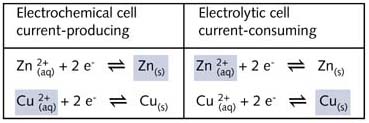
The constant external
supply of electricity forces the redox reaction to take place and in the example quoted
zinc ions are forced to oxidise the copper metal which in turn behaves as a reducing
agent.
In this electrolytic cell the zinc electrode acts as the cathode and the copper metal acts
as the anode. The cathode requires a constant supply of electrons which are available from
the electric current.
Cathodes and anodes
A cathode is the electrode at which reduction occurs. In an electrochemical cell it
receives electrons from the anode and has a positive sign, whereas in an electrolytic cell
it is the electrode at which cations attracted to it are reduced by the electrons supplied
to it. The cathode of an electrolytic cell has a negative sign due to electrons supplied
to it by the current source.
An anode is the electrode at which oxidation occurs. In an electrochemical cell it
supplies electrons to the cathode and has a negative sign, whereas in an electrolytic cell
it is the electrode at which anions attracted to it are oxidised. The anode of an
electrolytic cell has a positive sign due to its being connected to the positive pole of
the current source.

In electrochemical and electrolytic cells with the same redox couples in their half-cells,
such as depicted in illustration R11, the redox couples of the cathode and anode of the
electrochemical cell therefore become the redox couples of the anode and cathode
respectively of the electrolytic cell
Self-sustaining and non-self-sustaining redox reactions
In a
mixture ARed + BOx and AOx + BRed,
where ARed and AOx and BOx and BRed
are both conjugate redox couples, the following reactions are therefore possible:
1. Self-sustaining reactions proceeding to completion
![]()
The main requirement for a self-sustaining reaction is that there is a decrease in Gibbs
free energy due to the generation of heat a nd an increase in disorder. These conditions
are met for reactions proceeding from the stronger reducing agent and stronger oxidising
agent to the weaker reducing agent and weaker oxidising agent.
Strong reducing agents are to be found at the top righthand side of the table of redox
couple reduction potentials in illustration R7 and strong oxidising agents at the bottom
left-hand side of this table.
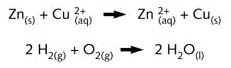
The half -reactions of the Daniell cell (see illustration R3 and R11) proceed to
completion without the use of a catalyst or the addition of external energy. This is also
true for direct mixing of the same reagents (see illustration R5), these reactions
continuing as long as there are reactants present.
The formation of water from hydrogen and oxygen can also be written with a heavy arrow
because this transformation only stops when at least one of the reagents is completely
used up. This reaction is, however, nonspontaneous, initiation either requiring a catalyst
or an increase in temperature of the reactants. Once the reaction has started, however, it
proceeds to completion without additional energy being supplied. This is an example of a
non-spontaneous self-sustaining redox reaction going to completion. These reactions both
proceed in the direction from the stronger reducing agent and stronger oxidising agent to
the weaker oxidising agent and weaker reducing agent (see illustration R10).
2. Self-sustaining equilibrium reactions
![]()
A redox reaction does not always proceed to completion despite proceeding from the
stronger oxidising agent and stronger reducing agents to the weaker ones. If the redox
half-reaction couples have very similar standard reduction potentials, the actual
reduction potentials of the redox half-reaction may converge as the reaction proceeds, the
reduction stopping when they become identical. In the half-reactions
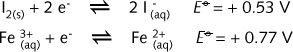
at certain concentrations the difference in reducing/oxidising capacity is so small, that the Gibbs free energy change involved gives rise to the possibility of a reaction in either direction. The system is said to have reached equilibrium. This is called a reversible equilibrium redox reaction and it can be represented by a double headed arrow.
![]()
This reaction is self-sustaining in both directions (no energy has to be supplied) and it
is spontaneous (no initial energy has to be provided). The system reaches equilibrium when
no further drop in Gibbs free energy is possible and not when one of the reagents has been
used up.
3.
Non-self-sustaining reactions
A redox reaction can, like any other reaction, occur in the reverse direction
until one of the reagents is used up. It is, for example, possible to
change copper metal back to copper ions by reacting it with zinc ions
which in turn become zinc metal. Such a redox reaction does not, however,
occur spontaneously and is never selfsustaining. An external energy supply
is required during the whole process. This is called a sustained redox
reaction and is accompanied by an increase in Gibbs free energy. In order
to predict whether two species – possibly after a short preparation
or with the help of a catalyst - can show a self-sustaining redox reaction,
we used the following practical rule: a species at the TOP RIGHT (in the
table) can in principle enter into such a redox reaction with a species
at the BOTTOM LEFT.
This
qualitative rule can also be expressed quantitatively in terms of the
electromotive force (e.m.f.) of the electrochemical cell made of the substances
written in these half cells: a self-sustaining redox reaction is only
possible if the e.m.f. of this cell turns out to be positive. For measuring
the electromotive force (e.m.f.) or cell potential (Ecell)
of an electrochemical cell, it is the convention to substract the reduction
potential of the anode from the reduction potential of the cathode. Not
all redox reactions can be sustained by supplying external energy. There
may be insurmountable kinetic and/or technical difficulties. A simple
way of supplying energy to a system is to apply an electric current i.e
a stream of electrons. Instead of producing energy as in an electrochemical
cell such as a Daniell cell, energy is supplied to a cell which is then
known as an electrolytic cell. Supply of external energy, the electric
current, gives rise to redox reactions which can be predicted by using
the table of standard reduction potentials in illustration R7. That way
it is possible to obtain zinc metal and Cu2+ by continuously
adding electrical energy to a "cell" of zinc ions and copper
metal. In such cases a weak oxidant (the most oxidised form from a more
reducing redox couple) must react with a weak reductor (the most reduced
form of a more oxidising half cell).a
more oxidising half cell).
Note :
The
voltage required from the external energy source is often greater than
that predicted by the difference, ![]() ,
of the two reduction potentials. Other reagents present in aqueous solution
can also be forced into a redox reaction by this large voltage. In the
example in illustration R11 sulphate ions
,
of the two reduction potentials. Other reagents present in aqueous solution
can also be forced into a redox reaction by this large voltage. In the
example in illustration R11 sulphate ions ![]() ,
are attracted towards the copper rod. The oxygen rather than the sulphur
from the
,
are attracted towards the copper rod. The oxygen rather than the sulphur
from the ![]() is oxidised,
oxygen gas being formed. This phenomenon can also be observed in the electrolysis
of water which has been acidified by H2SO4.
is oxidised,
oxygen gas being formed. This phenomenon can also be observed in the electrolysis
of water which has been acidified by H2SO4.