R10 Non-standard reduction potentials
Aim: To compare actual reduction potentials with standar reduction potentials for two very common half-reactions |
Illustration
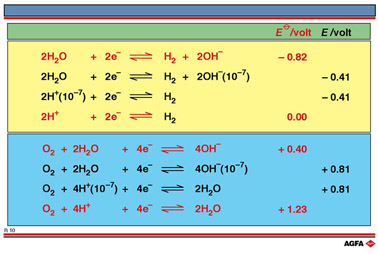
R10 shows
the ![]() and actual
reduction potential values, E, for the two half-reaction equations:
and actual
reduction potential values, E, for the two half-reaction equations:
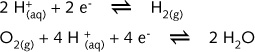
at various pH's
For each
half-reaction equation there are four different representations depending on the pH of the
medium in which they are to be found: two for bases (with OH-) and two with
acids (with H+). However only three E values are shown for each
half-reaction equation.
For the
top set of E values on illustration R10 i.e. the half-reaction 2H+/H2
:

The
middle values are not standard reduction potentials because the concentration
of H+ is not 1 mol/L and therefore the concentration is quoted
in the table. Lack of space prevents either the units or the physical
state of the reactants being quoted. Strictly speaking these should be
quoted as (g) (l) (s) (aq) depending on whether the reagent is a gas,
a liquid, a solid or an aqueous solution. The values given at the top
and bottom of the table are the standard reduction potentials for each
of the half-reactions. Fundamentally the process is the same, the reduction
of hydrogen, H, from oxidation number +I to 0. The ![]() values can be found in standard tables, but the non-standard reduction
potentials (middle values) are not. These can be calculated at 25°C for
the half-reaction 2H+/H2 using the Nernst-equation:
values can be found in standard tables, but the non-standard reduction
potentials (middle values) are not. These can be calculated at 25°C for
the half-reaction 2H+/H2 using the Nernst-equation:
![]()
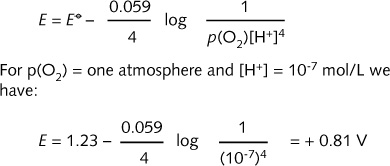
Thus :
![]()
For the bottom set of E values on illustration R10 i.e. the half-reaction O2/2H2O :

The process represents the reduction of oxygen from oxidation number 0 to -II. Again the
non-standard reduction potential can be calculated using the Nernst-equation.
Note :
Most
reactions in neutral aqueous solution are carried out at non-standard
reduction potentials. For the half-reaction H+/H2
the reduction potential used is -0.41 V and for the half-reaction O2/H2O
this is +0.81 V.
Illustration RR10 shows that the value does not change whether the half-reaction
is written with OH- or H+.
It can be concluded that hydrogen is a stronger reducing agent and oxygen
a stronger oxidising agent in water at pH 7 than under standard conditions.
In a neutral solution hydrogen ions, which are present in very small concentration,
are less likely to oxidise zinc metal than in an acid solution in which
their concentration is high.
This model can also be extended to consider oxygen gas in acid solution
in which it is more likely to oxidise iron than in a neutral solution
because the concentration of hydrogen ions is greater.