R09 Electrode-concentration cells
Aim: To show that a difference in concentration gives rise to a potential difference in a cell consisting of two otherwise identical hydrogen reference electrodes |
Illustration
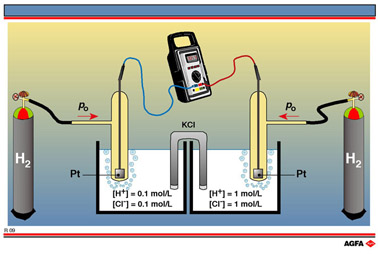
R9 shows
an electrochemical cell consisting of two hydrogen electrodes as half-cells,
only differing in the concentration of hydrochloric acid: 0.1 mol/L in
the half-cell on the left and 1 mol/L in the half-cell on the right. Hydrogen
is passed over both platinum electrodes at a pressure of 1 atmosphere
(1013 hPa) and the temperature is 25°C. The right-hand electrode is therefore
a standard hydrogen electrode and ![]() = 0 V . The pH of the left-hand half-cell is 1 (Concentration of H+
= 0.1 mol/L). The half-reaction equation for both half-cells is:
= 0 V . The pH of the left-hand half-cell is 1 (Concentration of H+
= 0.1 mol/L). The half-reaction equation for both half-cells is:
![]()
Which is
a 2 electron reaction with an expression for Qc of :
![]()
The voltmeter detects a potential difference between the two half-cells. This can only
result from the difference in concentration, since all other factors are identical The
actual reduction potential for the left-hand, non-standard half-cell reaction can be
determined using the Nernst equation:
![]()
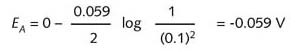
This value indicates that the left-hand half-cell is a stronger reducing agent than the
right-hand standard half-cell. The lower the concentration of hydrogen ions in the
left-hand half-cell , i.e. the higher the pH, the more negative the potential of the
left-hand half-cell. The potential for this cell, Ecell, is the difference
between the reduction potential of the cathode (right-hand half-cell) EC
and the reduction potential of the anode (left-hand half-cell), EA:
![]()
This
can be seen to have a positive value numerically equal to the value for
EA calculated using the Nernst equation. The cell convention
is to assign a positive value of Ecell to an electrochemical
cell-reaction when the reaction is written down in the direction of self-sustaining
change. A negative value for ![]() is required for a self-sustaining (spontaneous) reaction and since
is required for a self-sustaining (spontaneous) reaction and since ![]() = -nFEcell it is clear that if Ecell
is positive
= -nFEcell it is clear that if Ecell
is positive ![]() is
negative.
is
negative.
The fact that the potential difference between two otherwise identical
cells varies with pH is used in a potentiometric pH meter, despite the
fact that the hydrogen electrode is not well suited for routine pH measurement
since it is a source of gaseous hydrogen and is sensitive to various poisons
that inhibit the activity of the platinised surface of the electrode.
This potential difference can also be used to calculate an unknown concentration
of a solution using the same electrochemical cell as shown in illustration
R9.