R08 Concentration dependence of reduction potentials
|
Aim: To show the relationship between the actual reduction potential and the standard reduction potential and the concentrations of the reagents present. |
The standard reduction potential of a half-reaction equation when measured with respect to a standard hydrogen electrode is expressed in V. For a standard hydrogen electrode:
![]()
![]() = 0 V at a temperature
of 25 °C, a partial pressure of hydrogen of 1 atmosphere (1013 hPa) and
a concentration of H+ ions of 1 mol/L (i.e. pH = 0).
= 0 V at a temperature
of 25 °C, a partial pressure of hydrogen of 1 atmosphere (1013 hPa) and
a concentration of H+ ions of 1 mol/L (i.e. pH = 0).
The temperature, the partial pressure of gases, if the reagents are gases,
and the concentrations of the reagents involved in the half-reaction equation
have an effect on the electrochemical potential.
The first relationship between these factors was found by Nernst, a German
chemist and physicist, who in 1920
received a Nobel prize for his contributions to thermodynamics and electrolyte
solutions.
Nernst stated that:
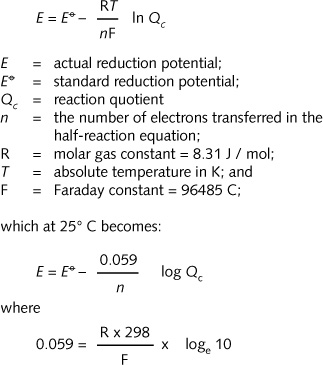
and
loge 10, the conversion factor from ln to log, is 2.3.
![]()
That
this is a 5 electron reduction reaction can be checked by considering
the change in the oxidation number of manganese from +VII in ![]() to +II in Mn2+ ions. The reaction quotient for this reaction,
to +II in Mn2+ ions. The reaction quotient for this reaction,
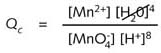
The
expression, Qc, should not be confused with the equilibrium
constant of a reaction which is always measured at equilibrium, the reaction
quotient only relating to the concentrations at a particular moment in
the reaction i.e. not necessarily at equilibrium.
The concentration
factor for the solvent, water , can be omitted from the equation since
it can be taken as 1 (see the Chemical equilibria module in DIDAC 2).
Thus at 25°C :
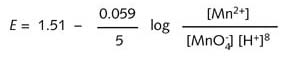
If
the respective concentrations are known then the actual value for E
at 25°C can be calculated.
It
can be seen from the above equation that E is dependent on the
concentration of hydrogen ions i.e. the acidity of the solution. As the
hydrogen ion concentration increases, i.e. pH decreases, E becomes
more positive i.e. the oxidising strength of the reducing agent increases.
Note :
For a pH-dependent reductor the reducing capacity increases (more negative E value) by keeping [H+] low or [OH-] high.
