R07 Table showing several standard reduction potentials
Aim: To show how, using a limited table of standard reduction potentials for redox half-reactions many experimental results can be explained and many more predicted |
Using illustration R5 it has been shown that the reducing power of redox couples i.e. the potentiality to donate electrons increases in the order:
![]()
and using illustration R6 it has been shown that the oxidising power and halogen redox couples i.e. the potentiality to accept electrons increases in the order:
![]()
this qualitative
information on the relative reducing and oxidising power of redox couples is quantified by
measuring their standard reduction potentials. Standard reduction potentials of
half-reactions are referred to the standard hydrogen electrode.
Standard
hydrogen electrode
The standard hydrogen electrode has, by convention, a standard reduction potential of zero volts at unit effective concentration, a temperature of 298 K and a hydrogen pressure of one atmosphere. The hydrogen electrode consists of a platinised platinum electrode immersed in a 1 mol/L solution hydrogen ions. Hydrogen gas at a pressure of 1 atm is bubbled over the platinum electrode. On the surface of the platinum an equilibrium is established between hydrogen gas and hydrogen ions.
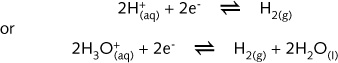
A potential develops on the surface of the platinum. It is assigned a value of zero volts.
Determination of standard reduction potentials
Standard reduction potentials are determined by connecting the system represented in the half-reaction equation concerned to a standard hydrogen electrode via a salt bridge and reading off the potential difference on either a previously calibrated potentiometer or a high resistance voltmeter. The standard hydrogen electrode is by convention always placed on the left.
The configuration of the cells whose standard reduction potential is being measured depends upon the species involved. In the case of a metal ion/metal couple the metal is present as an electrode immersed in a 1mol/L solution of its ions at 25° C. In the case of an ion/ion couple a platinum electrode is immersed in a solution containing 1 mol/L of each of the ions at 25° C. Finally, in the case of a gas/ion couple the gas at a pressure of 1 atm is bubbled over a platinum electrode immersed in a solution of 1 mol/L of ions at 25° C.
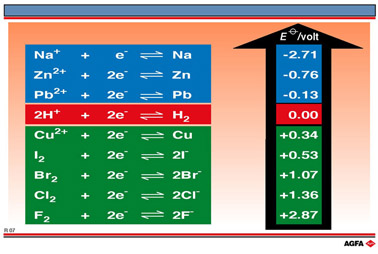
Table of standard reduction potentials
When
the values for ![]() are
tabulated it is the convention to write the most strongly reducing redox
couple at the top of the table and the most strongly oxidising couple
at the bottom.
are
tabulated it is the convention to write the most strongly reducing redox
couple at the top of the table and the most strongly oxidising couple
at the bottom.
he
table of standard reduction potentials in illustration R7 shows the
![]() values for
redox half-reactions which have been measured with respect to the standard
hydrogen electrode as reference.
values for
redox half-reactions which have been measured with respect to the standard
hydrogen electrode as reference.
It
will be noticed that the ![]() value of the redox half-reaction equations at the top of the table are
large and negative, while those for the half-reaction equations at the
bottom of the table are large and positive. Strong reducing agents and
hence the least oxidising redox couples, such as Na+/Na and
Zn2+/Zn have large negative
value of the redox half-reaction equations at the top of the table are
large and negative, while those for the half-reaction equations at the
bottom of the table are large and positive. Strong reducing agents and
hence the least oxidising redox couples, such as Na+/Na and
Zn2+/Zn have large negative ![]() values, whereas the least reducing and hence most oxidising redox couples
such as Cl2/2Cl- and F2/2F-
have large positive
values, whereas the least reducing and hence most oxidising redox couples
such as Cl2/2Cl- and F2/2F-
have large positive ![]() values and are to be found at the bottom of the table.
values and are to be found at the bottom of the table.
Use
of standard reduction potentials
This
table can be used to predict the outcome of classroom experiments and
also to explain various industrial processes which are based on redox
half-reaction equations by using their ![]() values.
values.
Bromine
is produced industrially by bubbling chlorine gas through a solution of
bromide ions. The same principle cannot be used to produce chlorine industrially,
because the installation necessary for the production of fluorine is too
expensive. Other oxidising agents such as the ![]() ,
which can be used in the laboratory scale production of chlorine, are
also too expensive for large scale use.
,
which can be used in the laboratory scale production of chlorine, are
also too expensive for large scale use.
Chlorine is produced industrially by the electrolysis of an aqueous solution
of sodium chloride. The process is not self-sustaining and electrical
energy must be constantly supplied. Electrolysis is the only method of
producing fluorine because there are no oxidising agents which are more
powerful than fluorine.
The table also clarifies why the alkali metals such as sodium (Na) react
so violently with the hydrogen ions in neutral water. The half-reaction
equations of these metals are above that of hydrogen ions and so they
reduce them. Zinc only reduces water in the presence of additional H+
ions, i.e. in an acid medium ( see illustration R9 for the influence of
ion concentration on reduction potentials).
The table also quantifies the qualitative difference in oxidising power of the Zn2+/Zn, Pb2+/Pb and Cu2+/Cu
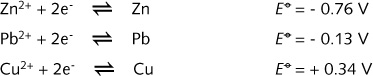
and quantifies
the redox reactions depicted in illustration R5. It is the convention
for measuring the cellpotential (Ecell) or electromotive force
(e.m.f.) of electrochemical cells always to place the more negative electrode
system (the anode) on the left :
This leads to :
![]()
because we substractthe reduction
potential of the left electrode from the reduction potential of the right
electrode.
On the basis of the standard half-cell redox potentials of Zn2+/Zn
and Cu2+/Cu one would expect Zn metal to play the role of the
anode (Zn oxidised to Zn2+) and Cu2+ the role of
the cathode (oxidising agent, being reduced).
Then :
![]()
This positive
value for Ecell ensures a self-sustaining redox reaction between
Zn and Cu2+, even when energy is not (continously) supplied
to the system.
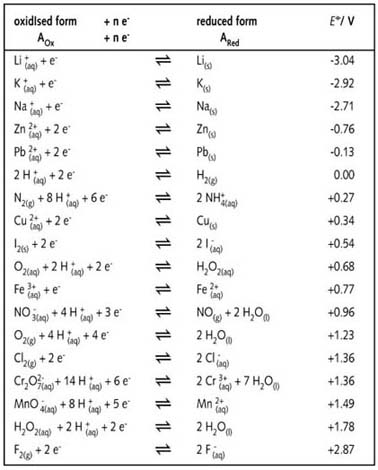
Important reducing and oxidising agents.
The table given below shows many more redox halfreaction equations than
have been considered in the preceding series of experiments. They have
been written in accordance with the IUPAC convention, all values are standard
reduction potentials and the letters (g) (l) (s) and (aq) have been added
to show the physical state: gas, liquid, solid and aqueous respectively.
These letters may be written on the same level as the symbol and immediately
next to it; e.g. Li+(aq) or ![]()
Compounds with both oxidising
and reducing properties
There are situations in which the ions indicated by a halfreaction equation
can function as both an oxidising and reducing agent depending on the
other ions in the reaction and the type of medium. Hydrogen peroxide can,
for example, function as both an oxidising and as a reducing agent. In
the table (left) the substance H2O2 is found top
right and bottom left :
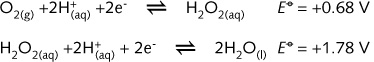
Simultaneous oxidation and reduction of a compound is known as disproportionation.
The fact that it can behave in this way does not affect neutral aqueous
hydrogen peroxide when being stored . Aqueous solutions only containing
H2O2 remain stable. Decomposition only occurs when
H2O2 comes into contact with a catalyst such as
Fe2+, Fe3+, I-, MnO2, blood, etc. It
is commonly used for cleaning contact lenses, bleaching hair and as a
disinfectant.
Ammonium nitrate (NH4NO3) is another compound that
can disproportionate, but this is not linked to the type of medium being
used and a real catalyst does not need to be present. This can be explained
by using the above table from which it can be seen that the ![]() ion is at the top right-hand side of the table and is therefore a strong
reducing agent. The nitrate ion (
ion is at the top right-hand side of the table and is therefore a strong
reducing agent. The nitrate ion (![]() )
is at the bottom left-hand side of the table and is therefore a possible
strong oxidising agent. In an aqueous solution or in the solid, the
)
is at the bottom left-hand side of the table and is therefore a possible
strong oxidising agent. In an aqueous solution or in the solid, the ![]() and
and ![]() ions
can remain stable.
ions
can remain stable.
A jolt however can be sufficient to start a disproportionation or autoxidoreduction
reactions.
Ammonium nitrate was used for many years as a fertilizer but it is also
a powerful explosive, decomposing on heating:
![]()
Ammonium dichromate (NH4)2Cr2O7(s)
also reacts in a similar way on heating decomposing spectacularly from
orange crystals to a large heap of green flakes.
Thermodynamic capability versus kinetic
reality
Above we were clearly confronted with examples of reductors and oxidants
which, even strongly mixed, do not proceed to an electron transfer or
a redox reaction.
Consider H2O2, that does not disintegrate spontaneously,
or NH4NO3, that does not explode spontaneously.
The fact that one reagent is an element to be found at the top right and
the other reagent is an element to be found at the bottom left of the
standard potential table does not always guarantee a self-sustaining redox
reaction.
Some cases require something more: an impulse, a push or a poke, in the
form of temperature or in the form of a catalyst. In this way H2O2,
which can safely be stored in (dark) bottles, will yet start to spontaneously
disintegrate as soon as a pinch of a catalysing substance is added to
it. Interesting to see here is the possible impact of iron (II) salts
and iron (III) salts: their half-reaction is somewhere between the two
half-reactions for hydrogen peroxide.
Useful as it may be, the table of standard potentials does not allow prediction
with certainty of which selfsustaining redox reactions are actually possible.
It is said that the table of standard potentials is normative for the
thermodynamic tendency of reductors and oxidants to react in a self-sustaining
way. No matter how big the difference between their E°-values
is, this does not say anything about the kinetics of the redox conversion,
i.e. the ease and the speed with which this can happen in practice.
A lack of visible reaction upon combination of redox couples from the
tables, which thermodynamically should produce a self-sustaining reaction,
may indicate a kinetic problem or could also indicate the formation of
an insoluble salt on the surface of the metal, especially if we think
of lead in an aqueous solution of zinc ions. The zinc salt should be choses
with care and zinc nitrate is probably the most suitable for use here.