R06 Table of half-reaction equation equations for non-metals
Aim: To arrange some halogen redox reactions according to their oxidation capacity |
At the top right-hand side
of the Periodic Table we find the halogen group; fluorine, chlorine, bromine and iodine.
They are normally found as diatomic molecules, X2.
The halogens (Greek for salt makers) are seldom found free in nature. This does not mean
that the bonding between the atoms to form diatomic molecules is weak, but is rather a
reflection of the fact that the halogens easily form ionic halides.
![]()
This is a redox reaction with a transfer of electrons taking place. Halogens
are well known oxidising agents, which indicates that the halogen/halide
ion redox couple has a potentiality for accepting electrons from another
reagents i.e. oxidising that agent.
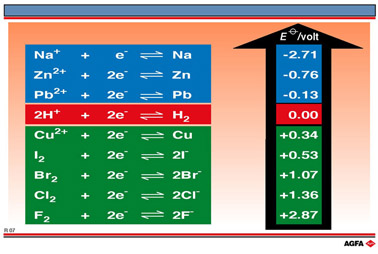
The potentiality of a particular X2/2X- redox couple
relative to other X2/2X- redox couples can be qualitatively
established by observing the effects of bringing different redox couples
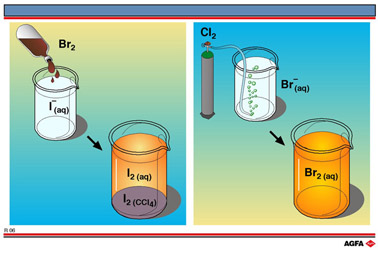
together. In illustration R6 two simple redox experiments are shown.
On the left-hand side of illustration R6 a brown liquid Br2
is mixed with an aqueous solution of iodide ions for example from potassium
iodide (KI). A light brown solution is obtained showing the presence of
a small quantity of iodine. To demonstrate the presence of iodine more
convincingly,a small volume of tetrachloromethane (CCl4) is
added and the vessel shaken. After leaving to stand for a few minutes
the bottom layer of CCl4 has a purple colour of I2,
while the aqueous layer has lost most of its brown colour. Another test
would be to add a few drops of starch solution, whereupon the presence
of a blueblack solution would indicate the presence of iodine. The total
reaction which is occurring can be represented as:
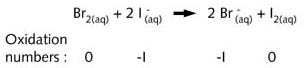
In this reaction Br2(aq)
has oxidised ![]() to I2(aq), while itself being reduced to
to I2(aq), while itself being reduced to ![]() .
Br2(aq) is therefore the oxidizing agent and
.
Br2(aq) is therefore the oxidizing agent and ![]() the reducing agent.
the reducing agent.
The Br2(aq)/2Br- half- reaction equation is the
stronger oxidiser and the two half-reaction equations can be written as
:
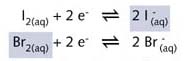
In the reaction
depicted on the right-hand side of illustration R6 chlorine (Cl2),
a light green gas,is
bubbled through a colourless aqueous solution of sodium bromide (NaBr).
A light brown aqueous solution of bromine results. Again the colour can
be intensified by shaking with a small volume of tetrachloromethane. Since
the bromine dissolves in CCl4 better than in water, the CCl4
layer at the bottom becomes brown. Bromine, like iodine, is apolar and
therefore dissolves better in the apolar CCl4 than in water
which is highly polar.
The reaction can be represented by two half-reaction equation equations:
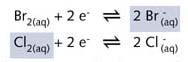
It is not possible
by means of such a simple reaction to show that fluorine reacts as an
oxidising agent with respect to chlorine, because it reacts violently
with water in a redox reaction during which a gas, HF, is produced.
I2, Br2, and Cl2, also react with water
but less violently. We may assume that a redox reaction between F2(aq)
as oxidising agent and ![]() as the reducing agent can be represented by the half-reaction equations:
as the reducing agent can be represented by the half-reaction equations:
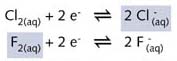
From these experiments it can be concluded that iodine is the least oxidising of the four halogen elements and that the oxidising power i.e. the potentiality of the X2/2X- couple to accept electrons increases in the order
iodine < bromine < chlorine < fluorine
Although these
simple experiments are not all carried out under the standard conditions
of temperature and pressure necessary for the measurements of standard
reduction potentials, they achieve their purpose of demonstrating convincingly
the order of their potentiality to accept electrons in the non-metallic
halogen group.
N.B.: When working with any halogen element all necessary safety precautions
should be taken.