R05 Ordering of half-reaction equations for metals
Aim: To arrange several metal redox couples according to their standard reduction potential |
A half-reaction equation represents a substance in equilibrium with an oxidised or reduced form of the same substance. According to IUPAC recommendations a halfreaction equation is always written in the form of a reduction with a substance on the left of the equation and a reduced form of the substance on the right of the equation. For example:
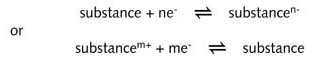
By
reduced form of a substance is meant a species in which an element is
characterised by a lower oxidation state than in the oxidised containing
the same element.
For a strip of copper in equilibrium with a solution of copper(II) ions
the half-reaction equation is:
![]()
Examples of half-reaction equations for a dissolved gas in equilibrium with its ions are:

These so-called redox couples have an ability spontaneously to donate
or accept electrons from another substance depending on their potentiality
for so doing.
The potentiality of a particular redox couple to donate or accept electrons
from another redox couple can be qualitatively established by observing
the effects of bringing different redox couples together in a redox reaction.
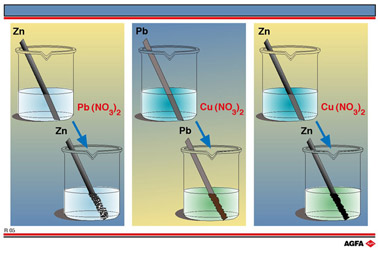
Let us consider the Zn2+/Zn, Pb2+/Pb and Cu2+/Cu redox couples. Illustration R5 shows three simple redox reactions. In the first a zinc rod in an aqueous solution of Pb(NO3)2 (lead(II) nitrate) reduces the lead(II) ions to lead, the oxidation number of lead changing from +II to 0. This is observed as a lead “tree” of dendritic lead crystals.
At the same time zinc is oxidised to zinc(II) ions, the oxidation number
of zinc changing from 0 to +II.

Adding these two half-reaction equations (top: right side; bottom : left side) so that no electrons are left, gives the overall redox reaction equation:
![]()
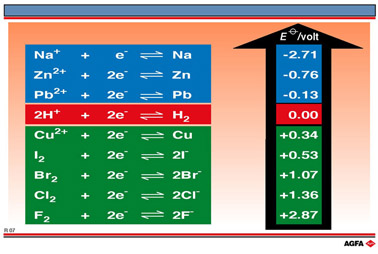
We can therefore conclude
that the Zn2+/Zn half-reaction equation is more strongly reducing than the Pb2+/Pb
half-reaction equation.
In the second experiment a lead rod reduces copper(II) ions to copper metal, the oxidation
number of copper changing from +II to 0. The blue colour of the aqueous solution is seen
to disappear. At the same time the lead is itself oxidised to lead(II) ions, the oxidation
number of lead changing from 0 to +II. The lead rod becomes brown in colour as copper is
deposited on it.

Adding the two half-reaction equations gives the overall redox reaction equation:
![]()
This shows that the lead
half-reaction equation is more strongly reducing than the copper half-reaction equation.
In the third experiment the zinc rod reduces the copper(II) ions to copper metal, the
oxidation number of copper changing from + II to 0, resulting in the original blue
solution becoming colourless and a red-brown deposit of copper being formed on the zinc
rod. At the same time the zinc is itself oxidised to zinc(II) ions, the oxidation number
of zinc changing from 0 to +II.

Adding the two half-reaction equations gives the overall redox reaction equation.
![]()
We can therefore conclude that the Zn2+/Zn half-reaction is more reducing than the Pb2+/Pb half-reaction, which in turn is more reducing than the Cu2+/Cu halfreaction equation.