R04
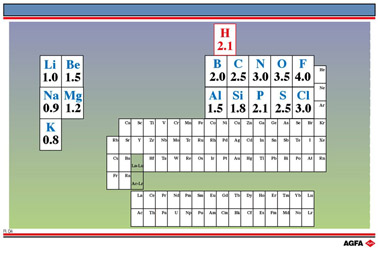
Table of electronegativity
|
Aim: To highlight the electronegativity of the elements in the description of redox reactions in general and of the oxidation number in particular |
The above-mentioned cases
concern metals and monoatomic metal ions among which an electron transfer took place. In
these cases, a complete exchange of electrons is evident.
A great deal of electron transfer reactions, however, concern uncharged covalent molecules
and/or multiatom ions. In the reaction which takes place in a gas cooker, methane reacts
with dioxygen, both covalent molecules. The reaction products CO2 and H2O
also belong to the group of exclusively covalent substances. Such combustion reactions can
essentially be reduced to a transfer of electrons between atoms as well.
This is easy to prove by letting both elements slowly react in a galvanic cell, in this
case a fuel cell. This cell actually produces electricity (electrical energy) from
chemical conversions (chemical energy).
![]()
Oxidation
number
In order to be able to describe such reactions as electron transfer reactions as well, we
have to follow accounting rules. Only then can chemists agree on the gain-loss balances of
electrons during a chemical conversion.
The cornerstone of this electron accounting is the socalled oxidation number, which is
assigned to a specific atom in a specific particle (ion or molecule):
the oxidation number (O.N.) of an individual atom in a specific particle is the electrical
charge in electron charge units actually carried by this atom, as an ion in an ionic bond,
or assigned to this atom by representing all covalent bonds as ionic bonds.
Oxidation numbers are assigned to atoms of a molecular structural formula showing all
valence electrons, on the basis of the following rules: all binding electrons in a
covalent bond are entirely displaced to the most electronegative partner (see R4). In the
case of equal electronegative partners, the binding electrons are equally distributed. The
amount of electrical charge (not in coulomb, but in electron charge units) assigned to the
atom is that obtained by the atom's group number less the number of valency electrons
remaining on this atom.
Since "the oxidation number of an atom" does not necessarily correspond with a
real ionic charge, such an O.N. is indicated by a Roman figure (instead of an Arabic
figure) preceded by + or - (instead of followed by the charge symbol). Referring to the
O.N. of an atom (in an ion or in a neutral molecule) one will therefore say, for instance,
+II (plus two) and not 2+ (two plus).
This means that the difference between O.N. and ionic charge is not only legible, but also
audible!
Examples :

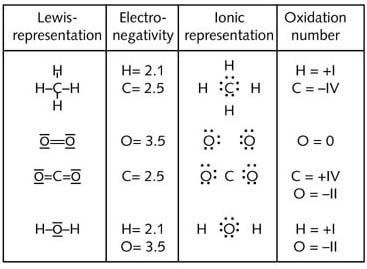
To be able
to unmistakably deduce the oxidation number of a covalently bonded atom,
the displacement of binding electrons (rule of play) has to be applied
to the molecule's or the ion's Lewis representation.
For I2 the Lewis notation becomes :![]() after
assignment of the binding electrons and the O.N.(I) will be 0, since the
group number of I is seven and the number of valency electrons in an ionic
representation of the covalent bond is seven as well:
after
assignment of the binding electrons and the O.N.(I) will be 0, since the
group number of I is seven and the number of valency electrons in an ionic
representation of the covalent bond is seven as well:
group number 7 - 7 valency electrons = 0.
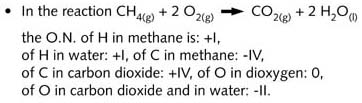
Below is a schematic diagram of the deduction of these oxidation numbers:
After the introduction
of the concept "oxidation number of an atom" it is understandable
that one began to use "oxidation-reduction reactions" or "redox
reactions" as general names for electron transfer reactions.
When in a particle the O.N of an atom increases due to the release of
(valency) electrons, it is said that this atom is being oxidized and that
the particle as a whole acts as a reductor. The released electrons will
be absorbed by an(other) atom into a(nother) particle. It is then said
that the atom is being reduced and that the particle as a whole acts as
an oxidant.
Important:
![]()
All electrons which are released by a reductor in a redox reaction
have to be absorbed by the oxidant. Therefore, the stoichiometric figures
of the oxidant and the reductor in a redox reaction have to be filled
in.
The symbols Red and Ox stand for "reduced form" and "oxidised
form" respectively.
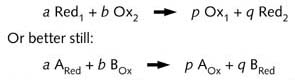
This is also valid for BOx or Ox2 and BRed or Red2
Further on, on illustration R5, we will see that this fundamental balance reaction, whether self-sustaining or not, mainly progresses from the direction of a relatively strong reductor and a relatively strong oxidant towards a weaker oxidant and a weaker reductor.
Certain techniques which are applied in class to deduce the stoichiometric values of a redox reaction are often based on the application of the oxidation number accounting. Nevertheless, the usual atom balance method is applicable to most redox reactions as well.
Electronegative values
The ability of an atom to attract the electrons of a covalent bond is measured by the electronegativity of the atom. An atom of high electronegativity will attract electrons away from one of lower electronegativity.
There are several electronegativity scales. In the Pauling electronegativity scale fluorine is given the value of 4.0 and the other elements are allotted values based on their electronegativities relative to fluorine.
Put in another way, the electronegativity of an element expresses on an arbitrary scale the relative attraction of one of its atoms for the electrons of a single covalent bond formed with an atom of another element.
Pauling electronegativities are given for selected elements in illustration R4.
Dealing with redox reactions we saw that the deduction of oxidation numbers is very important.
Therefore, a good ready
knowledge of the electronegative value of certain elements is just as important as the
knowledge of their electron configuration (number of valency electrons). Besides,
memorizing these values is very simple: For Li, 1.0 applies as Electronegative Value and
up to F an increment equal to 0.5 applies; from K to CI an increment equal to 0.3 applies,
except for the last two elements S and CI.
In this scale the Electronegative Value of H is 2.1.
Hydrogen is therefore not a metal, but a non-metal!