Bicyclopropylidene. A unique tetrasubstituted alkene and versatile
C6-building block for organic synthesis*
Armin de Meijere**, Sergei I. Kozhushkov, Thomas Späth,
Malte von Seebach, Sandra Löhr, Hanno Nüske, Tim Pohlmann,
Mazen Es-Sayed, and Stefan Bräse
Institut für Organische Chemie der Georg-August-Universität
Göttingen, Tammannstrasse 2, D-37077 Göttingen, Germany
Abstract: Bicyclopropylidene (4), now readily available
in preparatively viable quantities, is evolving as a useful C6
building block for organic synthesis due to its enhanced reactivity
at the C-H, the C=C, as well as both types of C-C single bonds. Monosubstituted
derivatives are accessible by deprotonation/electrophilic substitution.
Di- and tetrasubstituted bicyclopropylidenes are best made by copper-mediated
reductive dimerization of bromolithiocarbenoids. The 1,3-dipolar cycloadducts
of nitrones rearrange to spirocyclopropanated piperidones, palladium-catalyzed
codimerizations with acrylates occur with opening of one of the rings
to yield precursors to bicyclo[3.3.0]octene and bicyclo[5.3.0]decene
skeletons. Silicon-heteroatom bonds can be added across the double bond
of 4 under palladium catalysisjust like across a C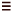 C
triple bond, and carbopalladation of the double bond in 4 occurs
more rapidly than that in an acrylate. A variety of new three-component
reactions of 4 with alkenyl as well as aryl halides and dienophiles
have been developed and extended to be carried out in a combinatorial
sense, even on a polymer support, with an additional dimension added
in the cleavage step. Most of the reported reactions of bicyclopropylidene
(4) proceed with good to excellent yields.
C
triple bond, and carbopalladation of the double bond in 4 occurs
more rapidly than that in an acrylate. A variety of new three-component
reactions of 4 with alkenyl as well as aryl halides and dienophiles
have been developed and extended to be carried out in a combinatorial
sense, even on a polymer support, with an additional dimension added
in the cleavage step. Most of the reported reactions of bicyclopropylidene
(4) proceed with good to excellent yields.
*Lecture presented at the 13th International
Conference on Organic Synthesis (ICOS-13), Warsaw, Poland, 1-5 July
2000.
** Corresponding author
Back to Contents for access to full text

