Title Core Modified N-confused and Expanded
Porphyrinoids: Syntheses, Characterization and Photodynamic Activity
Adviser Prof. T. K. Chandrashekar
Thesis Committee Prof. M. J. Therien, Department of Chemistry,
University of Pennsylvania, Philadelphia, USA; Prof. Martin Br�ring,
Institute of Inorganic Chemistry, University of W�rzburg, W�rzburg,
Germany; Prof. S. Mazumdar, Department of Chemical Sciences, Tata
Institute of Fundamental Research, India; Prof. A. J. Elias, Department
of Chemistry, IIT-Kanpur, India; Prof. A.Sharma, Department of Chemical
Engineering, IIT-Kanpur, India.
Essay
Porphyrins are the most widespread of all prosthetic
groups found in nature. These highly colored tetrapyrrolic macrocyclic
pigments play a diverse and critical role in biology ranging from
electron transfer, oxygen transport and storage, photosynthetic processes
and catalytic substrate oxidation which has inspired researchers around
the globe to and focus on these "pigments of life". Nature�s choice
of the porphyrin ring as the key structural backbone owes mainly to
its inherent conformational and redox flexibility, which provides
suitable coordination environment for many transition metals. The
interdisciplinary interest generated by the porphyrins resulted in
the syntheses of modified porphyrins, especially contracted, expanded
and isomeric porphyrinoids. The major objective of my doctoral research
was to formulate easy and efficient methodology to synthesize exotic
porphyrinoid macrocycles and study their electochemical, structural
and in a few cases their anion binding and photodynamic therapeutic
efficacy.
My dissertation was mainly devoted to the syntheses
and characterization of core modified N-confused and expanded porphyrins
(see fig. 1) as there were only few reports on such porphyrinoid macrocycles
bearing meso- aryl substituents. N-confused porphyrin is an isomer
of porphyrin, which has 18 p electrons in its aromatic pathway but
is associated with an inversion of one of the pyrrolic unit in the
macrocycle.
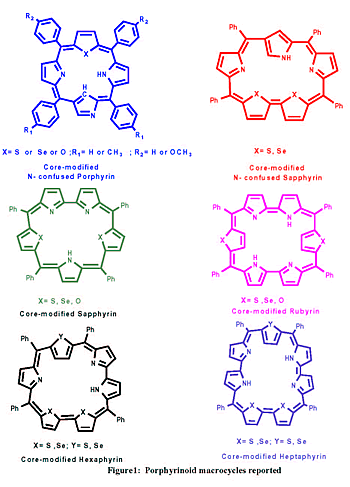
I have chosen expanded porphyrins, which results from
the expansion of the p electron conjugation by increasing the number
of heterocyclic rings as the objective mainly due to the synthetic
challenges encountered in previous attempts to make related macrocycles
in high yields. The resulting chromophores show strong absorptions
in the red region compared to normal 18p porphyrins. Core modification
of these expanded porphyrins changes the electronic structure of the
parent porphyrinoid, which in turn leads to interesting electrochemical,
magnetic and photochemical properties. Its inherent stability, photophysical,
electrochemical and biomedical applications have made it the cynosure
of attention in recent times. This motivated me to take up the challenge
of synthesizing fore mentioned porphyrinoids in high yields and evaluating
the structural diversity and bio-medical utility in detail.
After the serendipitous discovery of N-confused porphyrin
as a side product of meso-aryl porphyrin in 1994, attempts were on
for an improved synthetic methodology that will yield exclusive formation
of the desired isomer. My interest in exclusive syntheses of the core-modified
porphyrinoids encouraged me to introduce chalcogens into the existing
the N-confused porphyrin framework. Employing modified 3+1 MacDonald
condensation I successfully obtained products containing thiophene/furan/selenophene
units situated trans to the N-confused pyrrollic unit in high yields.
Detailed NMR studies at variable temperatures were done on these macrocycles
to establish the presence of three tautomers prevailing in solution.
The crystal structure for the thia derivative revealed a ruffled conformation
and a cyclophane like dimer formation in the unit cell due to the
presence of inter and intramolecular hydrogen bonds (fig.2). The electrochemical
and spectroscopic characteristics exhibited were interesting. The
syntheses of expanded porphyrin bearing an N-confused moiety can be
considered as a step forward in understanding the various interesting
properties exhibited by the expanded porphyrin initiated by alteration
of cavity size and electronic structure which can offer larger cavities
for the formation of 4d and 5d metal-carbon bonds. A perusal of literature
revealed that there are no reports on expanded porphyrin bearing N-confused
pyrrole. My experience with N-confused porphyrins prompted me to extend
the methodology used, to generate first examples of N-confused sapphyrins
through an easy and efficient 3+2 MacDonald condensation methodology.
In addition to synthesis, a detailed characterization of these modified
stable aromatic expanded porphyrins were done using 1 H and 2D NMR,
UV- visible spectroscopic techniques and single crystal X-ray structure
(refer fig. 2) determination to establish the inversion of the N-confused
ring in liquid and solid state. The formation of intra and intermolecular
hydrogen bonding in crystal packing, its ability to act as anion binding
agents in its diprotonated state were some of the interesting insights.
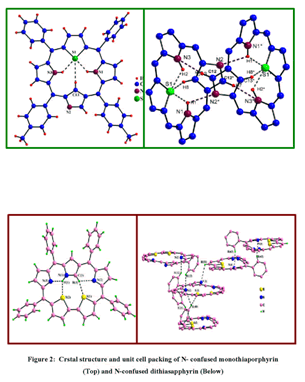
The simplest possible methodology till date to synthesize
core modified expanded porphyrins namely diheteroatom substituted
sapphyrins and rubyrins by condensation methodology involving heteroatom
containing diol with excess pyrrole in presence of protic acid catalyst
was also formulated during the course of reinvestigation of Ulman
methodology for the synthesis of core modified porphyrins. Moreover,
effect of natureand concentration acid catalyst used on the yields
of the resultant product distribution was followed carefully. 4+3
oxidative coupling reaction led to the syntheses of a range of hitherto
unknown expanded porphyrins, namely triheteroatom substituted [26]hexaphyrin(1.1.1.0.1.0),
which are isomers of rubyrin and [30]heptaphyrin(1.0.1.1.0.1.0) which
are the first aromatic meso-aryl heptaphyrins to be reported in the
literature. All the hexaphyrins and heptaphyrins reported possess
an inverted heterocyclic ring in their freebase and protonated state
and thus reveals structural diversity with respect to other reported
related macrocycles. Detailed studies on these conformational effects
were followed by geometry optimisation computational programmes, which
helped in establishing unequivocally the inverted geometry for the
heterocyclic ring opposite to the bithiophene/biselenophene moiety
in heptaphyrins and hexaphyrins. These differences highlight the presence
of subtle conformational effects in meso-aryl expanded porphyrinoids.
After constructive modifications of existing methodologies
and formulation of novel synthetic routes to arrive at these porphyrinoids,
my priority shifted further explore their efficiency as photosensitizer
in photodynamic therapy. Afore mentioned macrocycles absorb in the
red region compared to Photofrin, a hematoporphyrin derivative currently
used in the treatment of cancer. Preliminary studies of negligibly
dark toxic trithiasapphyrin based photosensitizer (fig.3) revealed
their efficiency to bind to human erythrocytes, which is a semi-model
system. The relative efficiency of the sensitizer calculated in terms
LC50 and LD50 based on time of incubation prior to exposure of light,
drug uptake studies and photohemolytic studies were found promising.
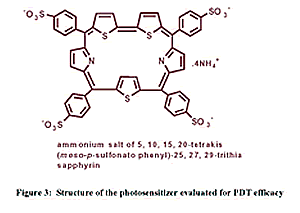
The simplicity of the high yielding reaction conditions
involving stable and variety of precursors containing heteroatoms
highlights the versatality of the synthetic methodology adopted. Thus
it is hoped that the availability of new methodology for high yield
syntheses of these new core modified expanded porphyrins will allow
further exploitation of their rich chemistry in terms of their coordination
behavior towards transition metals and their use as catalysts for
organic transformations and biomedical applications.

