Ph.D. Thesis
Title Remote Plasma Deposition of Hydrogenated
Amorphous Silicon: Plasma Processes, Film Growth, and Material Properties
Adviser Prof. M.C.M. van de Sanden
Thesis Committee D.C. Schram, Dept. of Applied Physics, Eindhoven
Univ. of Technology (NL); W.F. van der Weg, Dept. of Physics and Astronomy,
Utrecht Univ. (NL);. E.S. Aydil, Dept. of Chemical Engineering, Univ.
of California Santa Barbara (U.S.); N.J. Lopes Cardozo, FOM Institute
for Plasma Physics Rijnhuizen (NL); G.J.M. Meijer, FOM Institute for
Plasma Physics Rijnhuizen (NL); L.J.F. Hermans, Dept. of Physics,
Leiden Univ. (NL); W.J.M. de Jonge, Dept. of Applied Physics, Eindhoven
Univ. of Technology (NL); Niemantsverdriet, Dept. of Chemical Engineering,
Eindhoven Univ. of Technology (NL).
Essay
Electricity from solar cells is an important renewable
energy source to fulfill the worldwide growing energy demands while
preserving a healthy environment for future generations. Large-scale
introduction of solar cells will, however, require a considerable
number of technological breakthroughs, especially to reduce the cost
and to improve the efficiency of the cells. Among the different types
of cells, hydrogenated amorphous silicon (a-Si:H) solar cells are
one of the most promising candidates to enable this large-scale implementation.
In a-Si:H solar cells, electricity is generated by the absorption
of light in an alloy film of Si and H, which can be very thin (~500
nm) due to the high absorption caused by the film's non-crystalline
nature. This makes it possible to apply the cells on flexible foils
in a continuous roll-to-roll production process.
One of the main issues in this production process is
the increase of the deposition rate of the a-Si:H, which is usually
deposited by low-pressure plasma activation of silane (SiH4)
into reactive radicals and ions. Although a considerable part of the
thesis work has been devoted to an increase of the deposition rate
of device quality a-Si:H by a factor of 100 using a newly-developed
technique (the so-called "Expanding Thermal Plasma"), the fundamental
understanding of the deposition process is at least as important.
Insight into the plasma processes and the reactions taking place at
the film surface during growth and their relation to the film quality
is essential for full optimization of the a-Si:H production. Because
of the process's complexity, this thesis work focuses on using a broad
and integral approach to cover all the different aspects of the deposition
process of a-Si:H. Moreover, it yields very interesting science in
which different disciplines of research come together.
The first important aspect studied is the plasma chemistry
during deposition. Insight into the reactions taking place during
SiH4 dissociation and the species subsequently created
is crucial for the understanding of how the SiH4 is converted
into a film and how the plasma processes can be controlled by the
different plasma settings. These studies have been performed by investigations
of the densities of different silane radicals (SiHx,
x < or = 3) and cations SinHm+
in the aforementioned Ar-H2-SiH4 plasma. In
this remote plasma, SiH4 dissociation and deposition are
spatially separated, enabling not only high-rate deposition but also
independent parameter control. For the study of the low-density radical
species in the plasma several diagnostic techniques have been applied
such as optical emission spectroscopy (OES), threshold ionization
mass spectrometry (TIMS), and cavity ring down spectroscopy (CRDS).
Some of the diagnostics have particularly been adjusted for this purpose
to obtain high sensitivity under the harsh conditions of plasma deposition.
Among the different results obtained, it has been found that for a
considerable flow of ions from the plasma source, the SiH4
dissociation is governed by charge transfer reactions between these
ions and the SiH4. The created silane ions subsequently
undergo dominantly dissociative recombination with electrons leading
to hydrogen-deficient and consequently very reactive silane radicals
(SiHy, y < or = 2). Although the deposition process is dominated by
radicals under all conditions, a small fraction of the silane ions
will undergo sequential ion-molecule reactions with SiH4
forming large hydrogen-poor cationic clusters. It has even appeared
that larger clusters could be created in the plasma environment than
was theoretically predicted and measured under well-defined low-energy
conditions. Working at high H2 flows and therefore low
ion densities on the other hand, has revealed that SiH4
dissociation is governed by H abstraction reactions between atomic
H and SiH4 leading to SiH3 radicals. It has
been found that this is an important reaction for the deposition of
high quality a-Si:H as a considerable improvement of the film quality
has been observed with increasing contribution of SiH3
(Figure 1). This beneficial contribution of the SiH3 radical in terms
of film quality originates mainly from the low reactivity of the radical
as has been confirmed by surface reaction probability measurements.
SiH3 enables dense and defect-poor film growth as opposed
to the very hydrogen-deficient (poly)silane radicals that govern film
growth at low H2 flows. Furthermore, this work has revealed
the technologically relevant result that device quality a-Si:H can
be obtained at very high deposition rates when the a-Si:H film growth
is ~90% dominated by SiH3.
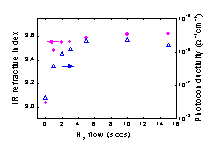
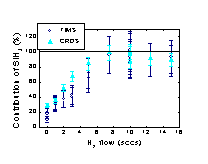
Fig.
1: The improvement of the a-Si:H film quality related to the increasing
contribution of the SiH3 radical to film growth.
In addition to the insight into the plasma processes,
knowledge of the nature of the a-Si:H surface during deposition is
crucial because at this interface the plasma species are converted
into film. Especially the hydrogen surface coverage and composition
is considered to have an important role. Therefore, a new in situ
technique for the analysis of the silicon surface hydrides during
deposition has been further developed and tested. In this technique,
attenuated total reflection infrared spectroscopy is combined with
ion-assisted hydrogen desorption to obtain surface selectivity. This
part of the thesis work, which was performed at the University of
California Santa Barbara, has revealed detailed information on ion-induced
reactive surface site creation and a thermally activated decomposition
reaction of higher surface hydrides into lower surface hydrides.
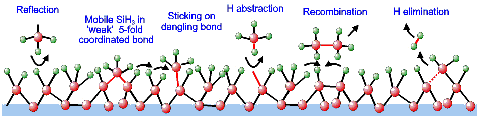
Finally, an extension of and improvement in the kinetic
growth model of a-Si:H has been proposed to gain a deeper understanding
of the reactions during growth on the atomic scale. This model is
fully based on surface reactions of SiH3 (Figure 2) as
justified by the above-mentioned results. With respect to SiH3's
low surface reactivity, the thermally activated decrease in surface
roughness, and the substrate temperature independent deposition rate
some important new insights are: the presence of mobile SiH3
on the surface in a "weaker" 5-fold coordinated bond in which the
SiH3 can subsequently diffuse to a dangling bond for strong
bond formation, surface dangling bond creation by direct H abstraction
by gaseous SiH3, and a hydrogen elimination process at
the surface. Especially the latter insight, which is based on the
surface infrared spectroscopy results, is a major accomplishment because
the riddle of how SiH3 containing 75% hydrogen can lead
to a film with only ~10% hydrogen has not yet been unraveled. The
hydrogen concentration has a large influence on the film's opto-electronic
properties and the observation that hydrogen elimination decreases
at high deposition rates might point out an important clue for high
rate deposition of high quality a-Si:H, i.e., finding a non-thermal
elimination process. This research has brought us one small step closer
to large-scale application of clean solar energy.