BP05 The structure of the peptide bond
Aim: To explain the possible configurations of the peptide bond and the peptide chain. |
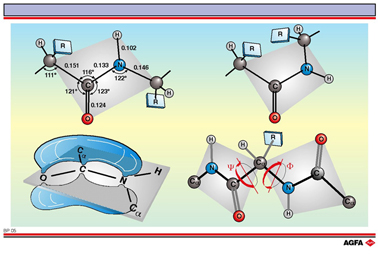
In the peptide bond the C=O and the N-H bonds are almost parallel to each other. In fact the oxygen, nitrogen, carbon and hydrogen atoms of the peptide bond and the neighbouring alpha-carbon atom find themselves in the same plane (shown in grey). The peptide bond is planar because the C-N bond has a substantial fraction of double bond characteristic. The peptide bond can be thought of as the resonant hybrid of two forms:
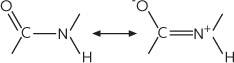
The left-hand figure on the
illustration gives a schematic depiction of the electron density in the peptide bond: the
ð electron cloud is delocalised over the O-C-N bonds. It follows that the flat peptide
bond allows two possible configurations. The trans-configuration (top left) is favoured
over the cis- (top right). In the cis-configuration the R-side-chains of neighbouring
alpha carbon atoms can have a strong sterical influence on each other.
The depiction of the trans-configuration contains the average bond lengths and angles for
the peptide bond.
Bond lengths are given in nanometers (nm).
When the secondary
structures are being formed the peptide chains can still make certain rotations around the
two bonds on the alpha-carbon atom in each amino acid residue. Theoretically many
secondary structures are possible since the rotation angles about this bond can vary.
They are defined as f and y structures. Sterically only a few
of these structures are possible.
The configuration depicted in the figure, below right, represents f =
+180° and y = +180°, an almost completely stretched
form of the polypeptide chain.