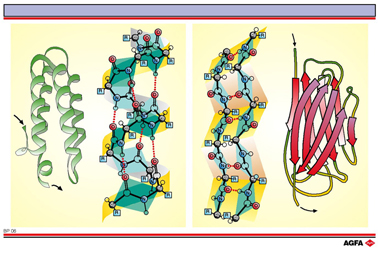
BP06 The a-helix and b-sheet
Aim: To characterise the two most important secondary structures. |
The two most
important secondary structures had already been suggested in 1951 by Linus
Pauling and Robert Carey: the ![]() -helix and the
-helix and the ![]() -sheet.
-sheet.
The
number of ![]() -helix domains that exist in
the secondary structure is determined by the nature of the R-side-chains
that point outwards. For example a proline residue cannot participate
in an
-helix domains that exist in
the secondary structure is determined by the nature of the R-side-chains
that point outwards. For example a proline residue cannot participate
in an ![]() -helix structure, and
the consecutive positioning of isoleucine residues (which are large R-side-groups)
would give rise to sterical problems and destabilise the formation of
an
-helix structure, and
the consecutive positioning of isoleucine residues (which are large R-side-groups)
would give rise to sterical problems and destabilise the formation of
an ![]() -helix.
-helix.
In the ![]() -sheet
(right) every residue is turned 180° with respect to the preceding one.
A number of neighbouring chains (in the figure just two have been drawn)
fold themselves in an accordion-like fashion, and intermolecular (between-the-chains)
hydrogen bonds hold the whole thing together. Schematically the
-sheet
(right) every residue is turned 180° with respect to the preceding one.
A number of neighbouring chains (in the figure just two have been drawn)
fold themselves in an accordion-like fashion, and intermolecular (between-the-chains)
hydrogen bonds hold the whole thing together. Schematically the
![]() -sheet is represented
by an arrow. The direction of the arrow indicates the N è C
direction of the polypeptide chain. If neighbouring chains run in the
same direction then we speak of a parallel
-sheet is represented
by an arrow. The direction of the arrow indicates the N è C
direction of the polypeptide chain. If neighbouring chains run in the
same direction then we speak of a parallel ![]() -sheet. If the neighbouring chains
run in opposite directions (as in the illustration) then we talk of an
anti-parallel
-sheet. If the neighbouring chains
run in opposite directions (as in the illustration) then we talk of an
anti-parallel ![]() -sheet.
-sheet.