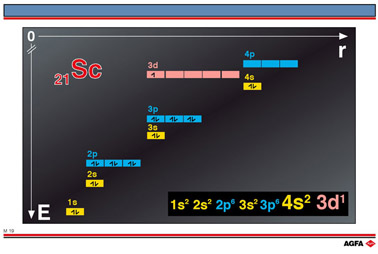
M19 Electronic configuration of scandium
Aim: To show how the orbital energy levels are filled for scandium |
In the case of scandium, 21Sc has 21 electrons filling the orbitals as follows:
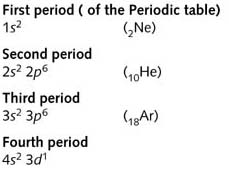
Note that the 4s level is lower than the 3d level