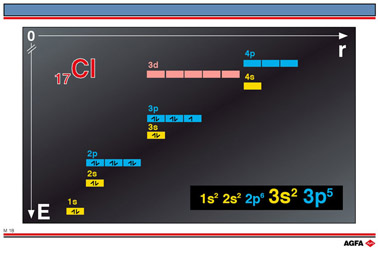
Aim: To show how
the orbital energy levels are filled for chlorine. |
|
|
For clarity only the energy levels up to 4p
are illustrated here. The orbital energy levels are filled with pairs of electrons
starting with the orbital with the lowest energy level, then the orbital (or orbitals)
with the next highest energy level and so on.
In the case of chlorine, 17Cl has 17 electrons filling the orbitals as follows:

|
