M17 The Aufbau principle and orbital energy levels
|
Aim: To represent the energy levels of the orbitals and explain the Aufbau principle |
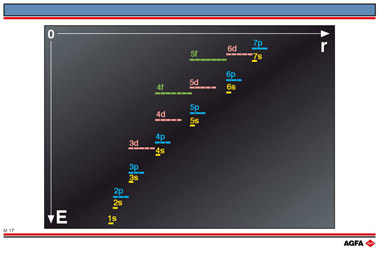
Illustration M17 shows the orbital energy levels as a function of r (the distance from the nucleus to the electron) or increasing principal quantum number, n, the integer associated with the energy levels e.g. 1s, 2p or 5f. The principle quantum number, n, is an integer, which determines the angular momentum, J, of electrons in permitted orbits, according to the expression:
![]()
[the integer
n in the expression describes the wavelengths of the series of spectral
lines emitted by hydrogen see comments regarding illustrations M09 and
M10)].
The energy of an electron in an atom can be characterised, by four quantum
numbers:
- the principal
quantum number, n, an integer;
- the subsidiary
quantum number, l, having an integral value from 0,1,2,3,….(n-1)
- the magnetic
quantum number, m, having an integral number varying from -l,
-(l-1), -(l-2),…0…(l-2), (l-1), l;
- the spin quantum number, s, having values of +1/2 and -1/2. The electron can be regarded as spinning on its axis, like a top, in a clockwise and anticlockwise direction.
This method of filling the orbitals’ energy levels was first proposed by Wolfgang Pauli (1900-1958). He stated in his “Exclusion Theory” that no two electron can have the same four quantum numbers. In other words no two electrons in any one atom behave in an identical manner. If the helium atom is considered in its lowest energy state, the two electrons are assigned the identical quantum numbers n=1, l=0 and m=0 which means that according to the Pauli exclusion principle the fourth quantum number, the spin number, must be different for the two electrons i.e. one has a spin of +1/2 and the other a spin of -1/2. A pairing of electrons with opposite spin is represented by the notation:
![]()
According to Hund’s
rule electrons occupy each level singly before electron pairing takes place.
According to the Aufbau principle the available electrons are filled in pairs in the
available orbitals in the order of increasing orbital energy level. For orbitals with the
same principle quantum number, n, e.g. 2s and 2p orbitals or 3s, 3p and 3d orbitals the
orbital energy level increases in the order: s< p< d< f.
All orbitals
of a particular shell or subshell (group of orbitals with the same principle
and subsidiary quantum number) e.g.; the 2p-orbitals, have the same energy.
The Aufbau principle is not always followed at the higher energy levels:
e.g. Ac: 7s2 6d1 instead of
7s2 5f1: the various energy levels overlap each
other.
References:
C.E. Mortimer, ‘Chemistry’, Wadsworth Publishing
Company, Belmont, California.