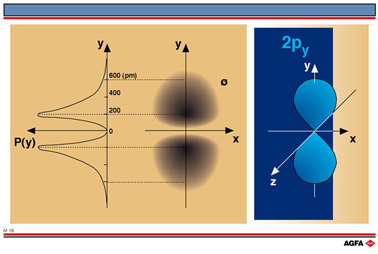
M16 2py-orbital
Aim: To show the spatial probability distribution of the py-orbital |
The electron density of the 2p-orbital 2p (n=2, l=1) is not spherical. Each p-orbital is concentrated along a particular axis, and consists of two parts (called lobes), one on either side of the nucleus. Since the axes of the orbitals are perpendicular to one another, there will be three identical orbitals : 2px , 2py , 2pz. On the left of illustration M16 the radial probability diagram of 2py orbital is shown. In the middle of the illustration the cross section of the electron density cloud is shown. It can be seen that the density gradually increases to a maximum and then decreases again with increasing distance from the nucleus.
Notes
Illustrations M14, M15, and M16 show the spatial probability distributions for the 1s, 2s,
and 2py orbitals respectively.
It is also possible to define directional probability distributions. These are obtained by
drawing vectors, originating at the centre of the nucleus, where the length of the vector
is proportional to the probability of finding an electron in a given direction. This
produces spheres for the 1s and 2s orbitals and dumb-bells (which do pass through the
origin) for the 2p orbitals.
References:
C.E.
Mortimer, ‘Chemistry’, Wadsworth Publishing
Company, Belmont, California.
J.E. Huheey, ‘Inorganic Chemistry, Principles of structure
and reactivity’, Harper International SI edition, New-
York.