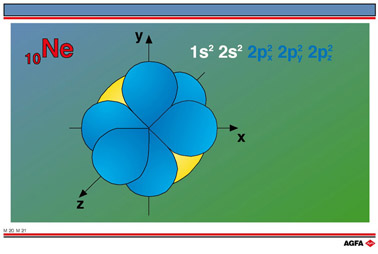
M20 - M21 Electronic configuration of néon
Aim: To show the s and p orbitals for neon, Ne, an inert gas |
The properties of inert or noble
gases are a result of their stable electronic configuration: for Ne full
s and p orbitals The orbital energy levels are filled with pairs of electrons
starting with the orbital with the lowest energy level, then the orbital
(or orbitals) with the next highest energy level and so on.
In the case of neon, 10Ne: 1s2 2s2
2p6 electrons filling the orbitals as follows:
electronic structure: 1s2 2s2 2p6.
Illustration M20-M21 represents the s and p orbitals. Since all three 2p orbitals are
full, the electron density is spherically symmetrical.