L16 Action of soaps and detergents
Aim: To show the structure of soap molecules and to show how they are remove apolar substances, such as fat, from the fibres of fabrics |
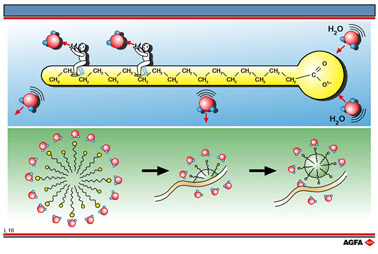
Soap is the sodium or potassium salt of a fatty acid. It is formed during the saponification of fats by sodium or potassium hydroxide. Apart from soap there are many other detergents all of which have the same general structure. All these molecules have a long hydrocarbon chain with at one end a highly polar carboxylate ion which is hydrophilic ( has an affinity for water) and lipophobic (oil and fat repellent ). At the other end is the long hydrocarbon chain which is hydrophobic (water repellent) and lipophilic (has an affinity for fats). Therefore, soap and detergent molecules are to be found between the water and oil layers.
The action of soap and detergent is identical. The dirt on clothing or on plates mainly consists of grease. This is insoluble in the washing water. When a detergent is added the molecules aggregate with the hydrophilic carboxylate ions on the outside and the hydrophobic hydrocarbon chains on the inside. The result is a very small spherical droplet which is called a micelle. These micelles do not coalesce because they are negatively charged due to the carboxylate ions on the surface and therefore repel one another. When micelles come into contact with fat the hydrocarbon chain in their core surround the fat and the micelles rearrange themselves with the hydrophilic carboxylate ions on the outside of the thereby emulsified fat in a so-called emulsification process.
Examples:

Detergents have the advantage over soaps that they form soluble calcium salts, whereas
soaps form insoluble salts, scum.
Detergents are often deactivated by the calcium ions in hard water which is why softeners
are added.