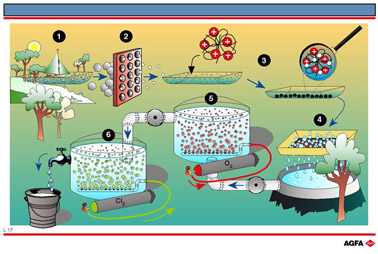
L17 Purification of drinking water
Aim: To show which processes are used to make drinking water from surface water. |
Drinking water is a
valuable commodity. It should therefore be used sparingly and it should not be wasted on
watering gardens, for which rain water should be used.
The following steps are involved in the provision of drinking water from surface water:
- Transportation from rivers, lakes
and reservoirs.
- Rapid filtration, usually through
a coarse sand filter, to remove large particles.
- Addition of a polyelectrolyte.
This is usually a polymer with a positive charge which acts as a flocculant
by absorbing small floating particles. These particles precipitate out
in the settling tanks. Aluminium sulphate can also be used.
- Slow filtration through a fine
sand filter to remove any remaining small particles. Sometimes the water
is also filtered through activated charcoal to remove organic molecules.
- Aeration of the water to oxidise
small quantities of organic material to carbon dioxide and water.
- Disinfection with either ozone or chlorine to render the remaining bacteria harmless and to reduce the growth of algae in water pipes. 3 to 6mg of chlorine per litre are added to the water with which it reacts to form hyperchlorous acid. This is a more efficient disinfectant than the hyperchlorous anion.
The increased use of fertilisers has led to an increased concentration of nitrates in the soil. The maximum acceptable concentration of nitrate in drinking water is 25mg/L in the US. Nitrates are harmful because they can be converted to nitrites and interfere with the assimilation of oxygen into the blood. Nitrates can be removed from water by reverse osmosis or reduced to nitrogen using particular strains of bacteria. These methods are only necessary when the maximum acceptable level of nitrate is exceeded.