L15 Water softeners : 2
Aim: To show how calcium ions can be removed from hard water : |
Softening of hard water entails the removal of calcium ions. This can be achieved: by ion exchange, precipitation or complex formation.
Phosphate
Pentasodiumtriphosphate (Na5P3O10) is a soluble salt of
tripolyphosphoric acid.
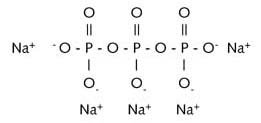
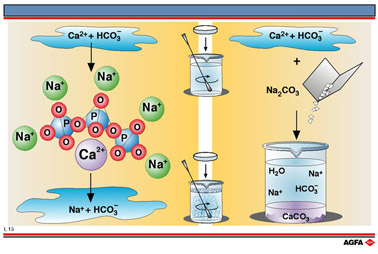
The anion ![]() forms an insoluble salt with calcium and magnesium ions. When phosphate
is added to hard water the complex formed is stable and does not decompose
on heating. The magnesium and calcium ions responsible for the hardness
are therefore no longer a problem when soap is added to water or water
is heated in a boiler. The calcium ions are , however, released if the
salt is subjected to prolonged heating at high temperatures.
forms an insoluble salt with calcium and magnesium ions. When phosphate
is added to hard water the complex formed is stable and does not decompose
on heating. The magnesium and calcium ions responsible for the hardness
are therefore no longer a problem when soap is added to water or water
is heated in a boiler. The calcium ions are , however, released if the
salt is subjected to prolonged heating at high temperatures.
The phosphate ions are not toxic, but in nature they are nutrients for plants. The phosphate levels in the rivers have increased substantially since phosphates were first added to washing powders. This has given rise to a lush and sometimes excessive plant growth in rivers and lakes (eutrophication, see illustration L18) since they are not biodegradable. Phosphates have, to a large extent, now been replaced by zeolites as water softeners in washing powders in order to avoid this problem. This, however, has led to another problem, that of excessive silt in rivers (see illustration L14).
Soda
Soda, sodium carbonat (Na2CO3) , can be used as a simple water
softener by simply exchanging ions with the calcium carbonate:
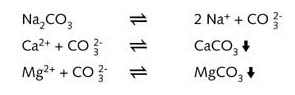
Calcium and magnesium ions precipitate out as insoluble carbonates and can no longer form
a scum with soap.
The water has thus been softened.
Bath salts often contain soda together with a perfume and a colouring agent. It also used
to be used to soften the water for the weekly wash.