L14 Water softeners : 1
Aim: To show how calcium ions can be removed from water |
Softening of water means the removal of the calcium ions which are responsible for the hardness. This can be achieved by ion exchange, precipitation or by complex formation.
Ion exchange
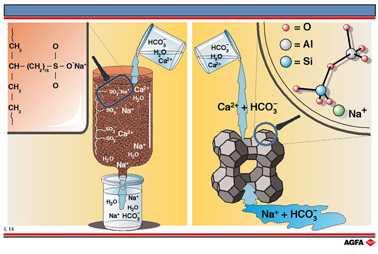
Ion exchange can be carried out with a cationic ion exchange resin or with a zeolite. A
cationic exchange resin consists of a resin with sulphate ion end-groups with a sodium
counterion. The sulphate groups bind calcium ions more strongly than sodium ions and
therefore as hard water flows through this resin the calcium ions, which cause the
hardness, are exchanged for sodium ions which do not cause hardness, thereby softening the
water. The resin eventually becomes saturated with calcium ions and has to be regenerated.
This can be done by pouring a concentrated solution of sodium chloride (Na-Cl, common
salt) through the resin during which the calcium ions are exchanged for the sodium ions.
This process is known as regeneration.
Zeolite
Zeolite can also be used for softening water, it being present in most modern washing
powders for this purpose. Zeolites are aluminosilicates and are formed when some of the Si4+
ions of an O-Si-O lattice structure are replaced by Al3+ ions . In the zeolite
A, that is used in washing powders, the ratio of silica to aluminium is 1:1.
Zeolites have a very open lattice built up of a series of tetrahedral unit cells. On the
four corners of each tetrahedron is an oxygen atom. These oxygen atoms surround either a
silicon or an aluminium atom. When silicon atoms with a valency of 4, are replaced by
aluminium atoms with a valency of 3, other cations, such as Na+ or Ca2+,
are required to preserve the charge neutrality.
These ions are incorporated in the cavities within the lattice structure.
When hard water is poured through zeolite containing sodium ions as compensating ions,
they are exchanged for the calcium ions from the water in the ratio of two Na+
for one Ca2+ and the water becomes softer.
Zeolites are solids but
when they are heated they appear to boil, hence the name boiling stone which is sometimes
attributed to them.
Zeolites, like other silicon salts, are fairly inert. Unlike phosphates, they are not
responsible for the clogging of surface water with algae, but they do produce a large
volume of silt which finishes up either in water-treatment plants or in rivers.