L13 Hardness of water
Aim: To show that hard water forms a scum with soap and has an adverse effect on heating elements.. |
There are two types of hard
water: temporary hard water which is caused by calcium hydrogen carbonate Ca(HCO3)2
dissolved in the water and permanent hard water which is caused by calcium and magnesium
sulphates dissolved in water. Temporary hardness can be removed by boiling, permanent
hardness cannot.
Hard water can therefore contain calcium ions (Ca2+), magnesium ions (Mg2+)
or both of these. The degree of hardness of tap water is mainly dependant on the
concentration of calcium ions in water.
When temporary hard water containing calcium hydrogen carbonate is heated this decomposes:
![]()
giving a deposit of insoluble calcium carbonate (CaCO3). This
precipitates out on the heating elements of kettles and boilers. As this
deposit becomes thicker ever more energy is needed to heat the water.
Water hardness can be expressed in several ways. It is assumed for the
purpose of illustration that hardness is only due to the calcium ion concentration.
- Molar
concentration of calcium ions per litre.
- ppm of
CaCO3
(1ppm CaCO3 =1mg CaCO3/ kg water = 0.01mmol Ca2+ ions /L.)
The hardness of water in the UK depends on the origin of the water, that originating from the granite hills of Scotland, for example, being soft whereas that originating from the limestone hills of the Pennines being hard.
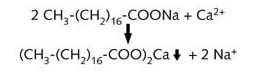
Hard water is not harmful to drink, but it furs up hot water pipes and boilers as well as increasing soap consumption.
