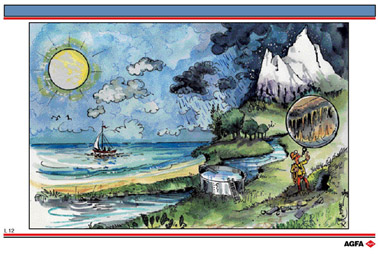
L12 The water cycle: formation of hard water
Aim: To show how calcium ions come to be in water |
The warmth of the sun causes a large amount of the water in the oceans to evaporate. The air becomes moist and is transported by the wind and when there is sufficient moisture to form water droplets or ice crystals clouds form. These droplets absorb carbon dioxide from the air and the water becomes slightly acidic.
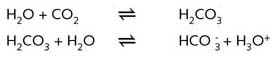
If this falls on sandy (silicate containing) soil very little happens except very gradual erosion of the sand. The water remains soft. If the rain falls on limestone then a chemical reaction occurs and the limestone is gradually dissolved away as calcium hydrogen carbonate forming underground caves.
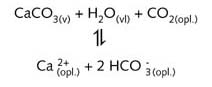
The water which flows through the limestone contains calcium ions and the water becomes
hard. (see illustration L13)
This water flows either
into the rivers or lakes or becomes ground water. Some of it flows back into the oceans
and a small amount is purified and used as drinking water.